Inorganic Flashcards
Describe an atom
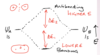
Atoms consist of a positively charged nucleus surrounded by negatively charged electrons. The nucleus is formed of positively charged protons and neutral neutrons.
Describe what determines an element
Elements are determined by their atomic number (Z) ie the number of protons they have in their nucleus.
Define atomic mass (A)
The number of protons + the number of neutrons
Describe isotopes
Isotopes are atoms of an element with the same number of protons but a different number of neutrons
State the mass of an electron relative to that of protons and neutrons
1/1836
Describe an electron
Early experiments showed electrons behaving as particles, with mass m and momentum p. They also have kinetic energy and a property called spin
State the equation relating momentum and mass
p = mv
p is momentum
m is mass
v is velocity
State the equation for kinetic energy
KE = 1/2mv2 m is the mass v is the velocity
Describe spin in an electron
Spin refers to the presence of a magnetic moment. For an electron the values spin can take are opposite, not parallel: ±1/2, or described as up and down
State De Broglie’s wavelength relationship
λ = h/p
λ is wavelength
h is planck’s constant
p is momentum mv (which demonstrates wave particle duality)
Give light’s fundamental properties and state the equation linking them
Wavelength (λ) frequency (f) and velocity (c)
λ x f = c
Give the equation for the energy of a photon, ie packet of light energy
E = hf
Describe the Rutherford model and why it has been superseded.
There was said to be a central massive nucleus orbited by electrons. Spectroscopy showed that only certain orbits were “allowed” which formed the basis for the Bohr model
Describe ᴪ (psi)
ᴪ represents wavefunction, which is a mathematical function describing the behaviour of an electron; it consists of a radial component and an angular component and describes the behaviour of an e- in an orbital. It refers to the amplitude of an electron
Describe what the Schrödinger equation is used for
The SE describes the behaviour of electrons in atoms, by treating them as waves. Ie, the SE is a wave equation
Describe solutions of the SE
Solutions of the SE are ᴪ (wavefunctions) which describe possible states for an electron.
Describe quantisation
Quantisation results directly from boundary conditions, which means only solutions and energies are possible
Describe ᴪ2
ᴪ2 refers to the probability of an electron being at a certain point, ie the electron density at a certain point.
State the three quantum numbers that can describe the 3D hydrogen orbital/ atom
n, l and ml
Define the lowest energy state
Lowest energy state is the ground state, and has the lowest value of the quantum number
State the name given to higher energy states
Excited states
Give the equation for wavenumber in which it is split into its radial and angular parts
ᴪ = R(r).Y(𝜃,ɸ)
r = radius
𝜃 (theta) = colatitude/angle defining orientation
ɸ (phi) = azimuth
Describe the radial wave function, R(r)
The radial wave function changes with distance from the nucleus - depends only on the radial distance between the nucleus and the electron. It depends on quantum numbers n and l, and contains no information on direction or orientation.
Describe the angular wave function, Y(𝜃,ɸ)
The angular wave function changes corresponding to different shapes - depends on direction or orientation but not distance, and on the quantum numbers l and ml. The angles 𝜃 and ɸ define a orientation with respect to a coordinate system.
Describe n; the principle quantum number
n describes the size of the orbital and can take any integral value from 1 to ∞. For species with just one electron (eg hydrogen atoms), the energy of the orbital depends on n but not l and ml. For any given n, energy order is s < p < d
Describe l; the angular momentum quantum number
l describes the shape of any orbital. It can take any integral value from 0 to n-1.
State which orbital has l=0
s orbital
State which orbital has l=1
p orbital
State which orbital has l=2
d orbital