Hospital acquired infection and antibiotic resistance Flashcards
Hospital acquired infections: list the reasons for the high rate of hospital acquired infection
- Presence of pathogens
- Very high density of very ill people – lots of pathogens
- Crowded wards
- People moving around – spreading (vectors)
- Open wounds – easy portal of entry
- Inserted medial devices – catheters, cannulas
- Antibiotic therapy (may suppress normal flora)
- Transmission by staff – contact with multiple patients
Antimicrobial mechanisms: summarise the mechanisms of action of important antimicrobials, and recognise that antimicrobial therapy provides a selection pressure for the spread of antimicrobial resistance
Antimicrobial; Any chemical/substance that kills a microbe or stops it’s growth
- Bactericidal – kills bacteria.
- Bacteriostatic – stops bacteria growing.
- Antiseptic – chemical that kills or inhibits microbes that is usually used topically to prevent infection.
“Selection pressure for the spread of antimicrobial resistance”
A resistant strain of bacteria appears shortly after the introduction of the antimicrobial
Important classes:
-
Beta-lactams:
- Inhibit cell wall (peptidoglycan) synthesis
- Penicillin, methicillin (bind to penicillin-binding proteins which catalyze synthesis of peptidoglycan)
- bactericidal
- Penicillin resistance works via drug inactivation (beta-lactamase) or altered target site
-
Tetracycline:
- Inhibits protein synthesis
- Bacteriostatic
- Tetracycline resistance is mediated by efflux/membrane pumps
-
Chloramphenicol:
- Inhibit protein synthesis
- Bacteriostatic but with Higher toxicity
-
Sulphonamides:
- Sulpha-methoxazole
- Bacteriostatic and are used in Combination therapy
- ex. Protonsil (treats UTIs, bacteremia and prophylaxis for HIV+ individuals)
- Sulfonamide resistance is conferred by blocking uptake/decreasing influx.
-
Aminoglycosides:
- Bactericidal
- Inhibit protein synthesis and cause damage to cell membrane
- Toxicity has limited use, but resistance to other ABs has led to increasing use
- Gentamicin, streptomycin
Quinolones:
- Gram +ve infections
- Inhibits protein synthesis
- Synthetic, broad spectrum, bactericidal.
- Target DNA gyrase in Gm-ve and topoisomerase IV in Gm+ve.
- Resistance is associated with target site modification
- bactereicidal
1. Macrolides:- Gram +ve infections (mainly)
- Erythromycin, azithromycin.
- Inhibits protein synthesis by targeting 50s ribosomal subunits
- both bacteriostatic and bactericidal
Specific antibiotics examples:
- Rifampicin
- Bactericidal.
- Targets RpoB subunit of RNA polymerase.
- Spontaneous resistance is frequent.
- Makes secretions go orange/red – affects compliance.
- Vancomycin
- Bactericidal
- Targets Lipid II component of cell wall biosynthesis, as well as wall crosslinking via D-ala residues
- Toxicity has limited use, but resistance to other antibiotics has led to increasing use e.g. against MRSA
- Daptomycin
- Bactericidal.
- Targets bacterial cell membrane.
- Gram-positive spectrum of activity.
- Toxicity limits dose
- Linezolid
- Bacteriostatic.
- Inhibits the initiation of protein synthesis by binding to the 50S rRNA subunit.
- Gram-positive spectrum of activity
Antimicrobial resistance: summarise the mechanisms of antimicrobial resistance, list important bacterial pathogens that are multi-drug resistant, and explain why antimicrobial resistance is associated with increased morbidity, mortality, length of hospital stay and cost
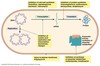
Simple explanation of Antimicrobial resistance
- Colony of bacteria – some are resistant, some aren’t
- Antibiotics given
- Non – resistant are wiped out
- Only left with resistant bacteria
- No resource competition
- Resistant strains flourish
Sources of antibiotic resistance genes
- Plasmids – extra-chromosomal circular DNA, often multiple copy. Often carry mutliple AB res genes – selection for one maintains resistance to all.
- Transposons. Integrate into chromosomal DNA. Allow transfer of genes from plasmid to chromosome and vice versa.
- Naked DNA. DNA from dead bacteria released into environment.
Mechanism – 4 pathways:
- Altered target site;
- Antibiotic can no longer bind to target site
- Can arise via acquisition of alternative gene or a gene that encodes a target-modifying enzyme
- Methicillin-resistant Staphylococcus aureus (MRSA) encodes an alternative PBP (PBP2a) with low affinity for beta-lactams.
- Antibiotic inactivation; enzymatic degradation or alteration of the antibiotic before it has an effect. (ex, b-lactamase resistant to penicillin)
- Altered metabolism; Increased substrate production outcompetes antibiotic target mechanism (e.g. increased production of PABA confers resistance to sulfonamides).or can switch to new metabolic pathways
- Decreased drug accumulation; Decreased entry or increased efflux of antibiotic and Infectious dose is not reached
Morbidity – more exposure to hospital pathogens
Mortality – more exposure to hospital pathogens
Length of stay – more exposure to hospital pathogens, more illness
Cost –
- more drugs, cost of bed stay
- increased time to effective therapy
- Requirement for additional approaches – e.g. surgery.
- Use of expensive therapy (newer drugs).
- Use of more toxic drugs e.g. vancomycin.
- Use of less effective ‘second choice’ antibiotics
Multi drug resistant bacteria
- Gram -ve
- E. Coli
- Salmonella
- Neisseria gonorrhoeae
- Pseudomonas aeruginosa
- Gram +ve
- Staphylococcus Aureus (MRSA)
- Streptococcus pneumoniae
- Clostridium difficile
- Mycobacteria
- Mycobacterium tuberculosis
Treatment: summarise the main approaches used to prevent hospital acquired infections and the emergence of drug-resistant bacteria
Strategies;
- Temporarily withdraw certain classes, tighter controls, use for only dangerous infections
- Reduce use of broad-spectrum antibiotics
- Identify resistant strains quicker
- Combination therapy
- Chemically adjust current antibiotics


