Hematopathology Flashcards

This is the appearance of normal bone marrow at medium magnification. Note the presence of megakaryocytes, erythroid islands, and granulocytic precursors. This marrow is taken from the posterior iliac crest in a middle aged person, so it is about 50% cellular, with steatocytes admixed with the marrow elements.

This is the appearance of normal bone marrow smear at high magnification. Note the presence of an eosinophilic myelocyte, a basophilic myelocyte, and a plasma cell.

Normochromasia
The red blood cells here are normal, happy RBC’s. They have a zone of central pallor about 1/3 the size of the RBC. The RBC’s demonstrate minimal variation in size (anisocytosis) and shape (poikilocytosis). A few small fuzzy blue platelets are seen. In the center of the field are a band neutrophil on the left and a segmented neutrophil on the right.

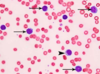
A normal mature lymphocyte with a single large nucleus is seen on the left, compared to a segmented neutrophil on the right with multiple nuclear lobes connected by thin chromatin bridges, along with cytoplasmic granules. An RBC is seen to be about 2/3 the size of a normal lymphocyte.
Though granulocytes survive a matter of hours, lymphocytes survive weeks, or even months. They may enter and exit the circulation to reside in places such as lymphoid tissues.

The RBC’s in the background appear normal. The important finding here is the presence of many PMN’s. An elevated WBC count with mainly neutrophils suggests inflammation or infection. This neutrophilia increases the total WBC count. This neutrophilia consists of segmented neutrophils and band neutrophils, but not eosinophils or basophils.

An elevated WBC count with segmented neutrophils as well as more immature band neutrophils and even metamyelocytes suggests more severe inflammation or infection driving accelerated release of myeloid cells from the bone marrow. This is known as a ‘left shift’. A very high WBC count (>50,000) with pronounced left shift that is not a leukemia is known as a “leukemoid reaction”. This reaction can be distinguished from malignant WBC’s by the presence of large amounts of leukocyte alkaline phosphatase (LAP) in the normal neutrophils.
WBC count & differential


The RBC’s here have stacked together in long chains. This is known as “rouleaux formation” and it happens with increased serum proteins, particularly fibrinogen and globulins. Such long chains of RBC’s sediment more readily. This is the mechanism for the sedimentation rate, which increases non-specifically with inflammation and increased “acute phase” serum proteins [CRP, SAA, fibrinogen, haptoglobin; activated by IL1 and IL6]
Anisocytosis
Variation in RBC size
Poikilocytosis
a condition where 10% or more of the red blood cells are abnormally shaped due to other medical conditions
Anemia in disease
Inflammatory conditions release cytokines such as interleukins 1 and 6 (IL1, IL6) that stimulate hepatic production of hepcidin,
During conditions in which the hepcidin level is abnormally high, such as inflammation, serum iron falls due to iron trapping within macrophages and liver cells and decreased gut iron absorption. This typically leads to anemia due to an inadequate amount of serum iron being available for developing red blood cells. When the hepcidin level is abnormally low such as in hemochromatosis, iron overload occurs due to increased ferroportin mediated iron efflux from storage and increased gut iron absorption.
Hepcidin induction by inflammation is presumed to have evolved to sequester iron from pathogenic microorganisms.
The result is decreased total serum iron, but iron binding capacity is reduced as well, resulting in a somewhat decreased saturation, but increased ferritin. Serum soluble transferrin receptors will be unaffected by chronic disease states. Anemia of chronic disease is addressed by treating the underlying condition.
Transferrin
glycoproteins found in vertebrates which bind to and consequently mediate the transport of Iron (Fe) through blood plasma. They are produced in the liver and contain binding sites for two Fe3+ ions. Human transferrin is encoded by the TF gene and produced as a 76 kDa glycoprotein.
Transferrin glycoproteins bind iron tightly, but reversibly

Hematopoietic elements in this bone marrow biopsy are markedly reduced. This is a case of aplastic anemia. Of course, besides, RBC’s the platelets and granulocytes will often be diminished. Sometimes a drug or toxin is the cause and sometimes infection. When no known cause can be found, it is termed idiopathic aplastic anemia.

In contrast to aplastic anemia, leukemia results in a highly cellular marrow. The marrow between the pink bone trabeculae seen here is nearly 100% cellular, and it consists of leukemic cells of acute lymphocytic leukemia (ALL) that have virtually replaced or suppressed normal hematopoiesis. Thus, though the marrow is quite cellular, there can be peripheral cytopenias. This explains the complications of infection (lack of normal leukocytes), hemorrhage (lack of platelets), and anemia (lack of red blood cells) that often appear with leukemia.

The WBC’s seen here are lymphocytes, but they are blasts–very immature cells with scant cytoplasm and large nuclei that contain nucleoli. Such abnormal lymphocytes are indicative of acute lymphoblastic leukemia (ALL). ALL is more common in children than adults. Many cases of ALL in children respond well to treatment, and many are curable.

These mature lymphocytes are increased markedly in number. They are indicative of chronic lymphocytic leukemia, a disease most often seen in older adults. This disease responds poorly to treatment, but it is indolent.
CLL is defined by more than 5000/microliter B lymphocytes in peripheral blood marking with CD23 and CD5. Over half of patients are diagnosed at an early, asymptomatic stage, without lymphadenopathy, splenomegaly, cytopenias, or autoimmune phenomena. With counts >10,000/microliter, progression and severity of CLL become more likely.
Though CLL is a B cell proliferation, marking with CD19 and CD20, it is characterized by the presence of a T cell marker, CD5, as shown by flow cytometry here. This is a systemic disease, and organ involement outside of marrow, such as spleen and liver, is known as small lymphocytic lymphoma (SLL).

Here are very large, immature myeloblasts with many nucleoli. A distincitve feature of these blasts is a linear red “Auer rod” composed of crystallized granules. These findings are typical for acute myelogenous leukemia (AML) that is most prevalent in young adults.

Leukemias typically fill up the marrow with abnormal cells, displacing normal hematopoiesis. The marrow here is essentially 100% cellular, but composed almost exclusively of leukemic cells. Normal hematopoiesis is reduced via replacement (a “myelophthisic” process) or by suppressed stem cell division. Thus, leukemic patients are prone to anemia, thrombocytopenia, and granulocytopenia and all of the complications that ensue, particularly complications of bleeding and infection.