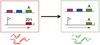
HAT and Immune Invasion - 2a Flashcards
Antigen
molecule that triggers production of a specific antibody
T. brucei is highly susceptible to
antibodies
- lives in bloodstream - fully exposed to antibodies response
- induce STRONG antibody response
- antibodies are effective at clearing this pathogen
Immune invasion
- number of trypanosomes found in blood is NOT constant
- waves of parasitemia
- difference between parasitemia peaks 5-7 days
Waves of parasitemia
- each wave represents an antigenically distinct serotype (clone
- parasites are clonal within peaks
- parasites are antigenically distinct in different waves
- antibodies produced in the 1st week will not react with parasites generated in the second week
so antibodies don’t recognize later peaks
correlation - parasite numbers with wave of fever
(wave of host temperature just after parasite peak)
(parasites being killed and releasing contents that are also antigenic - our immune system responds to these lysis products = fever)
Change in antigen profile is called
antigenic variation
Antigenic variation
(picture)
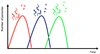

red parasite increases in number
→ antibodies generated against red
→ red parasite decreases in number
some red parasites change molecule on cell surface that is revealed to the immune system
→ grow and elicit an immune response
→ decrease in numbers
always looking to stay one step ahead of our immune system
Antigenic variation
- entire population in host appears uniform
- at low frequency (1 in 1million cells) some cells have a different serotype (SWITCHING)
- T. brucei has 1500 genes to choose from, plus can recombine those genes
The process of switching
antigenic variation
Surface of T. brucei cell covered with
electron dense coat
- protein that covers the surface of the cell and the flagella
- antisera reacts strongly with surface coat
- surface coats from different clones are antigenically distinct
- varies among different parasites and in different ways
Antisera reacts strongly with
surface coat
Surface coats from different clones are
antigenically distinct
Trypsin
- a protease treatment that completely removes the surface coat from trypanosomes
- trypsin treatment stops antibody binding
- implies that antigenic variation is caused by a surface protein
The electron-dense coat is made of
Variant Surface Glycoproten (VSG)
- surface coat is made up almost of a single VSG
- VSG is highly immunogenic
- electron-dense coat stimulates immune system
- VSGs from different parasitemia peaks differ in their amino acid sequences
- only 1 VSG being expressed by 1 parasite in a population at a time
- different waves have different VSGs
VSGs are
homodimers that are split into 4 regions
- 10 million per cell
- ~65 kDa glycoprotein
- 10% total cell protein
VSG structure

Signal sequence
~20 amino acids
Variable domain
~360 amino acids
distinguished the different types of VSGs
Conserved region
~100 amino acids
Hydrophobic sequence
~20 amino acids
cDNA sequence indicates VSG has
extension at N- and C- termini
- in actual protein this sequence is missing
why the difference
- post-translational modification
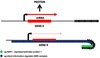
VSG amino terminal
(picture)

VSG amino terminal
- VSG mRNA translated into protein sequence that’s being trafficked into the ER
- protein into ER
- the 20 amino acid signal sequence at the amino terminal acts as an ER signal
- once the ER signal is within the ER lumen it undergoes proteolysis and is clipped off
- protein translation carries on until the hydrophobic domain is produced
- this hydrophobic domain acts as an anchor
- so the protein is synthesized, most ends up toward ER lumen
- the hydrophobic terminal at the C terminus of the protein captures and holds the pole of the protein onto the membrane surface
- the hydrophobic end of the protein interacts with hydrophobic bilayer of the ER
- that hydrophobic sequence is clipped off but the protein remains bound to the hydrophobic membrane
- the protein is clipped and the hydrophobic domain is replaced with a glycolipid sugar fat moiety
- post-translational modification at its C-terminus
- changed a string of amino acids to a sugar-fat moiety
- this sugar-fat moiety is referred to as the GPI anchor
VSG amino terminal (sum)
- VSG enters the ER
- C-terminal
- hydrophobic
- binds VSG to the ER membrane
- cleaved off
- replaced with a glycolipid (sugar/fat)
- covalently linked
GPI anchor
glycolipid (sugar/fat)
- consists of core sugar (4) residues + phosphatidylinositol phospholipid
- sugar component can be branched
- called glycosylphosphatidylinositol (GPI) anchor
way to anchor VSG to membrane without protein/peptide sequence
GPI anchor
(picture)
- blue = phosphatidylinositol phospholipid
- green = sugar component (can be branched)

VSG molecules dimerize
- form a homodimer
- 2 GPI anchors - 1 from each monomer - anchoring it to the membrane
- conserved domains come together - lots of cystiene molecules that can form disulfide bonds within the conserved domains
- protein then transferred via secretory pathway to cell surface
VSGs covering the cell surface
ER → golgi → vesicles (clathrin coated) → flagella pocket (vesicles fuse via exocytosis) and vesicles form part of the membrane of the flagella pocket, expose VSGs to extracellular environment in flagella pocket, fluid mosaic model in flagella pocket come out of pocket → cover cell surface
VSG in flagella pocket, comes out → covers surface