Half life Flashcards
As an isotope decays, the number of nuclei of that isotope that remain will ___________.
As a consequence of this, the _________ of that isotope will also decrease over time
Decrease
Activity
The half-life of an isotope
is the time taken for the activity of that isotope (or the number of original nuclei) to drop to half of its initial value
Every time one half-life passes, the activity (and the number of nuclei) will fall by half
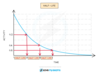
Every time one half-life passes
the activity halves
or
the number of radioactive nuclei half.
The activity (and number of nuclei) will never quite drop to zero
(T/F)
True

An isotope has an initial activity of 120 Bq.
6 days later it’s activity is 15 Bg.
The number of half-lives that have passed is:
120/2 = 60
60/2 = 30
30/2 = 15
We had to halve 120 three times to get to 15, and so three half-lives have passed.
Therefore each half-life must be:
6 days/3 = 2 days
Background Radiation
Background radiation is radiation that is always present in the environment around us
As a consequence, whenever an experiment involving radiation is carried out, some of the radiation that is detected will be background radiation
When carrying out experiments to measure half-life, the presence of background radiation must be taken into account
Accounting for background radiation in half-life calculations
Start by measuring background radiation (with no sources present) – this is called your background count
Then carry out your experiment
Subtract the background count from each of your readings, in order to give a corrected count
The corrected count is your best estimate of the radiation emitted from the source, and should be used to measure its half-life



