Glycoaminoglycans Flashcards
What are GAG? proteoglycans? glycoproteins?
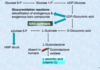
- GAGs: Negatively charged heteropolysaccharides
- Proteoglycans: Carbohydrates (GAGs; >95%) + Proteins
- Glycoproteins: Carbohydrates(small amount) + Proteins
- Long, unbranched polysaccharides containing a repeating disaccharide unit. [acidic sugar-amino sugar]n
- Disaccharide units contain
-
Amino sugars
- N-acetylglucosamine (GlcNAc) or
- N-acetylgalactosamine (GalNAc)
** - Acidic sugar **
- Uronic acids
- Glucuronic acid
- Iduronic acid
What are the features and functions of gag?
- Negatively charged and bind to large amount of water (hydrated); extended in solution
- Produce gel-like matrix- forms the basis of ground substance which along with fibrous structural proteins forms the extracellular matrix (ECM)
- Hydrated GAGs provide flexible support to the ECM
- Acts as a molecular sieve in ECM
- “Slippery” consistency of mucous secretions & synovial fluid due to the due to the large number of negative charges on GAGs; they repel each other & slide past each other
- When GAGs are compressed, water is ‘squeezed out’; when compression is released, GAGs spring back to their original hydrated volume
- Contributes to the resilience of synovial fluid and the vitreous humor of the eye
What are the types of GAG ( mucopolysaccrides) and where are they located?
- Located primarily
- On the surface of cells
- In the extra cellular matrix (ECM)
- Mucus secretions - Types:
- Hyaluronic acid
- Dermatan sulfate
- Chondroitin sulfate
- Keratan sulfate
- Heparan sulfate
- Heparin
HYANDURONIC ACID
Composition: D-glucuronic acid + N-acetyl glucosamine
(GlcNAC)
- Non sulfated & Not covalently linked to proteins
- Location: Synovial fluid, vitreous humor, umbilical cord
ECM of loose connective tissue
Functions: Lubricant and shock absorber, role in cell migration during embryogenesis
Dermatan sulfate
Composition: L-iduronic acid + N-acetyl galactosamine 4-S
(GalNAc)
- Location: Skin, blood vessels, heart valves
- Functions: Constituent of skin, role in wound healing
Chondroitin 4- and 6-sulfates
Composition: D-glucuronic acid + GalNAc-4- or 6- sulfate
- Form proteoglycan aggregates with Hyaluronic acid
- Most abundant GAG in the body
- Location: Cartilage, tendons, ligaments, aorta, cornea
- Function: In cartilage it binds to collagen and hold fibers in a tight strong network
- Composition: D-glucuronate-2-sulfate (10%)
(or iduronate-2-sulfate (90%) \+ N-sulfo-D-glucosamine-6-sulfate - (heparans have less sulfate than heparins)
Ø Heparin: - Only intracellular GAG àmast cells lining arteries in liver, lungs and skin
- Anticoagulant: Heparin activates antithrombin III, which in turn inhibits thrombin & other clotting factors
Heparan sulfate
Ø: Basement membrane and cell surfaces
It binds specifically to lipoprotein lipase present in capillary walls
Karatin sulfate
- Composition: Galactose + GlcNAc-6-sulfate
No Uronic acid
- Most heterogeneous as may also contain L-Fucose,
N-acetyl neuraminic acid (NANA) & mannose
- Location: Cornea, bone, cartilage aggregated with Chondroitin sulfates
- Function: In cornea both Keratan sulfate & dermatan sulfate lie between collagen fibrils & facilitate corneal transparency.
Explain the structure of proteoglycans
The protein cores of proteoglycans are rich in Serine & threonine residues, which allows multiple GAG attachments
üMany such Proteoglycans monomers aggregate on a molecule of Hyaluronic acid to form proteoglycans aggregates through ionic interactions and stabilized by linker proteins
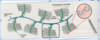
Explain how the the GAG is attached to the protein core
GAGs extend perpendicularly from the core in a bottle brush-like structure
Linkage of GAGs to protein core involves a specific trisaccharide, two galactose residues and a xylulose residue
(GAG—GalGalXyl-O-CH2-protein).
The trisaccharide linker is coupled to the protein core through an O-glycosidic bond to a Serine residue in the protein

Explain the synthesis of glucuronic acid for GAG formation

Where isthe synthesis of GAG, what are the amino and acidic sugars involved? Where does the synthesis of the core protein take place?
- Synthesis of GAG: Golgi, glycosyltransferase
- Amino sugars (amino group donated by glutamine)
- N-acetyl glucosamine & N-acetyl galactosamine
- Acidic sugars
- D-Glucuronic acid & L-Iduronic acid
- Glucuronic acid synthesized by uronic acid pathway
- (3’phosphoadenosyl-5’-phosphosulfate) PAPS is the donor of sulfate group
- Amino sugars (amino group donated by glutamine)
- Synthesis of core protein:
- RER
What is the disease associated with the synthesis of GAG and proteoglycans?
Chondrodystrophies:
- Autosomal Recessive
- Defect in the sulfation of GAG chain
- Improper development and maintenance of the
skeletal system
How are GAG’s degraded?
Degradation of GAG: Lysosomes, Acid Hydrolases
Extra cellular GAG
¯
phagocytosed
¯
fused with a lysosome
¯
endoglycosidases
¯
desulfated & deacetylated
¯
further action of acid hydrolases