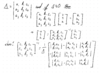General Flashcards
Fourier Transform
This is a way to describe an oscillating function in terms of angle and intensity by changing the function to be a function of angular frequency (rad/sec) or wavenumber (k=seconds to complete a wave).
It uses eulers identity to switch the imaginary parts of the frequency into cos and sine terms. This is then converted into polar coordinates in the intensity phase space.
Use the table to convert regular functions in fourier equations that are used to analyze a frequency.
In the manual the period is given so 2 pi over the angular frequency give syou seconds and the functions for several common signal inputs.

Even and odd functions
f(-x)=f(x) means the function is even (x2 cos)
f(-x)=-f(x) means that it is odd (x3 sin)

Orthogonal functions
This is when the integral of f(x)*g(x)=0
Taylor Series
Taylor Series represent using a series of bisected functions to model an original function f(x). They converge when |x-a|

Fourier transform of a cosine wave
This will yield one line because each and every interval is on the same frequency which means your peak will show that one frequency.
Fourier transform of a series of signals with t(duration)=tau
This will yield several bumps because thetime domain shows 2 intensities within one 360 degree rotation.
Integers, rational, and irrational numbers
Integers are whole numbers
Rational numbers are numbers that can be written as a ratio of two integers
irrational numbers cannot be expressed as a ratio of two integers. They include numbers that repeat or do not terminate Ex: (3)^.5/2
This is irrational because 3^.5 has an operator
Real numbers: include all of the above
factorial
n!=(n)*(n-1)*…(2)*(1)
Complex Numbers (Add, Subtract, multiply, divide)
Add/subtract: combine like terms.
Mult.: FOIL
Divide: Multiply by the conjugate of the denominator
Using conjugates
if A=a+ib and A#=a-ib then A*A#=a2 + b2
Sets
A set is a collection of objects or entities. If X belongs to a set S then X ∈ S where S={n0,n1…n} or a set could be defined by a function S={X:X3>27 }
Joining Sets
∈
C ∪ D = {X:X∈C or X∈D) This is the union of sets C and D
C ∩ D = {X:X∈C and X∈D) This is the intersection of sets C and D
And if all of a set (set A) are part of a larger set (set B) then AcB where the c is the inclusion symbol
Ø
This is the null set symbol. It means that there are not any elements within a set.
Exponent Laws
n√a=a(1/n)
am/an= am-n am*an= am+n
(am)n=am*n
(a*b)m= am*bm
Simplify n √am
n √am= a(1/n)*m = a (m/n)
Simplify n √ab
n √ab= a(1/n) * b(1/n)
How to take the root of a function with a divisor
Multiply the denominator through and divide the whole term by the diviseor with the root operator used.
Rule for binomial expansion
if we have a binomial given by (A+X)n then the expansion is given by B*An-m * Xm
Where: m=term # starting from 0 to n+1
B=(The exponent*coefficient of the term m-1)/m
Ex: Term 3 of (A+X)7 is given by A7+7A6X+(7*6/2)A5X2…
Determinants
Determinants are the values of matrices. The order of a determinant is given by the sqaure root of the number of elements which are the numbers inside of the matrix.
determinant nomenclature
the position of any value is given by aij where i= row # and j = collumn #
Finding determinants of second order matrix
If the determinant is second order then the determinant is given by:
Product of primary diagonal-Product of secondary diagonal
Where: Primary diagonal are the values given by a11 and a22 and the secondary diagonal is given by the values a21 and a12
Minor of a matrix
This is the reduced matrix that can be used to find the determinant of higher order matrices. The order is given by crossing out the values that intersect at the value of interest and finding the determinant of the resulting matrix multiplied by the value of interest.
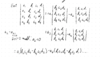
Sign of determinant parts
If the leading coefficient is in the position ij where i+j is
even then the coefficient is positive
odd then the coefficient is negative
Solving Linear Equations with two unknowns
when given a set of equations like
a1x+b1y=c1 and a2x+b2y=c2
Multiply the the first equation by -a2 and the second by a1 so that when added the first terms cancel. This enables you to have only one unknown to be solved for.
y=(-a2c1+a2c2)/(a1b2-a2b1) and x=(c1b2-c2b1)/(a1b2-a2b1)