Gases Flashcards
On Earth, in what states does matter exist?
Exists in three solid physical states: solid, liquid,gases
Gas Laws:
This law states that pressure and volume of a gas are inversely proportional.
Boyle`s Law

Gas Laws: states that the volume of a given mass of an ideal gas increases or decreases by the same factor as its temperature at constant pressure,
Charles’ Law
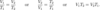
Charles did V-T experiments on various gases and found that regardless of the gas used the x-intercept of the V-T graph was always -273°C.What is…
- the significance of this number?
- The name?
- who gave this number meaning?
-273.15°C is called absolute zero
Lord Kelvin interpretated that to mean that at that temperature all molecular motion would cease–the kinetic energy and the volume of the gas would be 0 .
what is the equation to convert celsius to kelvins?

Gas Laws: The pressure of a gas of fixed mass and fixed volume is directly proportional to the gas’ absolute temperature.
Gay-Lussac’s Law

What is STP and its values?
STP= Standard temperature and pressure
- 0°C / 273.15K
- 101.3 kPa
What is SATP and its values?
Standard Ambien temp and pressure
- 25°C / 298.15 K
- 100.0 kPa
What is the Combined Gas Law?

This law states that the total pressure of a mixture of non-reacting gases is equal to the sum of the partial pressures of the individual gases.
Dalton’s Law of Partial Pressures
“The force that is exerted on an object per unit of surface area.”
Pressure
The equation to calculate pressure is:
where:
- p= pressure
- F=force
- A=area

What are the two main factors in determining the state of the substance?
- the attractive forces holding the particles together
- the kinetic energy of the particles that tends to pull them apart
What makes a gas ideal?
- have mass
- no volume
- no attractive force
- have high translational kinetic energy
What is standard atmospheric pressure?
atmospheric pressure in dry air at a temperature of 0ºC at sea level

