Functional Groups & pKa Flashcards
1
Q

A
carboxyllic acid
pKa = 4
2
Q

A
ketone
pKa = 20-24
3
Q

A
thiol
pKa = 13
4
Q

A
Alkane
pKa = 50
5
Q

A
acid chloride
6
Q

A
Alkyne
pKa = 25
7
Q

A
epoxide
8
Q

A
amine
pKa = 35
9
Q

A
Alcohol
pKa = 17
10
Q

A
ether
11
Q

A
disulfide
12
Q

A
imine
13
Q

A
sulfide
14
Q

A
nitrile
pKa = 25
15
Q
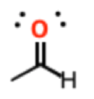
A
aldehyde
pKa = 20-24
16
Q

A
amide
17
Q

A
ester
pKa = 25
18
Q

A
Alkene
pKa = 43
19
Q

A
anhydride
20
Q

A
nitro
21
Q

A
alkyl halide
22
Q

A
phenyl
(benzene ring)
23
Q

A
hydroiodic acid
pKa = -10
24
Q

A
hydrobromic acid
pka = -9
25

malonates
pka = 13
26

water
pka = 16
27

hydrogen
pka = 36
28

sulfoxide
pka = 31
29

protenated amines
pka = 9-11
30

sulfonic acids
pka = -1
31

hydroflouric acid
pka = 3.2
32

hydronium ion
pka = -1.7
33

sulfuric acid
pka = -3
34

Hydrochloric acid
pKa = -3


