exam 3 Flashcards
1
Q
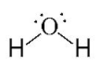
A
Tetra Hedral Bent
104.5
2
Q

A
Linear Linear
180
3
Q

A
Octahedral Octahedral
90
4
Q

A
Trigonal Bypyrimadil Seesaw
90
5
Q

A
Trigonal Bypyrimidal T Shaped
90
6
Q

A
Trigonal Bypyrimidal Linear
180
7
Q
A
Trigonal Planar Bent
120
8
Q
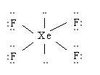
A
Octahedral Square Planar
90
9
Q

A
Tetrahedral Tetrahedral
109.5
10
Q

A
Trigonal bypyrimidal Trigonal Bypyrimidal
120
109.5
11
Q

A
Tetrahedral Trigonal Pyrimidal
107
12
Q

A
Trigonal Planar Trigonal Planar
122
13
Q

A
Octahedral Square Pyrimidal
90
14
Q
Atomic Radii increase which way on the columns of the periodic table?
A
Down
15
Q
Atomic Radii increase which way on the rows of the periodic table?
A
Left


