Exam 1 Flashcards
(205 cards)
3 aspects of internal validity before statistical association:
-check for bias, counfounding/effect modification, statistical significance
Bias
systematic (non-random) error in study design or conduct leading to erroneous results (distorts relationship btw exposure & outcome)
after study end- fix bias?
Nothing you can do to fix it
prospective (prestudy)
adjustment minimize bias (assess to confirm internal validity and conclusions)
components of bias
- source 2. magnitude 3. direction
bias magnitude
how much of an impact bias has on changing odds ratio
bias direction
move Odds Ratio/Risk Ratio away or towards 1.0 (groups equal) enhance or minimizing
main categories of bias
how you measure (collect data) or Selection (ppl) -measurement- related biases -selection-related biases * both result in grps different–dont want– want grps to ne as equal as possible except the one thing you are studying biases make grps different & creates the error
measurement- related biases
way the researcher collects information, or measures/observes subjects which created a systemic difference between groups in quality/accuracy of info.
selection-related biases
way the researcher selects or acquires study subjects which creates a systemic difference in the composition between groups (not from same pop/group)
types of selection bias
health-worker bias self-selection/participant (res ponder) bias control selection bias
selection bias
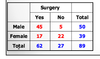
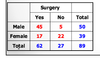
-selecting study subjects that are not representative of your primary pop. of interest or generates differences in grps being compared -how you pick your people- bias– made groups different (inappropriate) -convenience study: mall on wed.
health-worker bias
environmental employer research -sick & dead not at job site
self-selection/participant (responded) bias
people who want to be in survey -volunteer different than nonresponse
control selection bias
only diff btw grps not clear based on disease definition -call 1st 50, not accounting for if they have a phone/can they answer
recall/reporting bias
subject-related variation -differential level of accuracy/detail in info btw grps - ex: recall after bad event, after disease; diabetics monitor eating more than regular ppl
hawthorne effect
overly encouraging, give more than needed, overly positive/helpful
types of measurement bias
subject related: recall bias, contamination bias, compliance bias, lost to follow up bias observer related: interviewer bias, diagnosis bias,
contamination bias
aspirin v placebo: dont want other drugs in same family (how investigators account for what else taking) -control grp accidentally receive tx of exposed to intervention being studied
compliance/adherence bias
comply, follow instructions- worry one grp different in complying -grps being interventionally studied have different compliances
lost to follow-up bias (attrition)
follow up diff in 1 grp or another; diff withdrawal or lost to follow-up rates or other differences -differential v. non-differential ex: surgery-good pain control drugs vs. placebo– hear from person in pain or drops out
interviewer (proficiency) bias
- not trained in what to do -interviewer expects certain answers -conscious or unconscious –> mask inverstigator so they dont know who meds/who placebo -body language, inflection, expect diff. answers- skewed/false info
diagnosis/surveilance (expection) bias
diff evlauation, classification, diagnosis, observation preconceived expectations (hawthorne-like effects from researchers) expect drug to feel better
controlling for biases
*Select precise, accurate, & medically-appropriate measures of assessment and evaluation/observation -validated screening -specifics of data collection ex: polygraph test *blinging/masking *multiple sources data *randomly allocate observers for data collection *methods to minimize loss to follow up