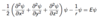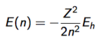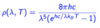Equations Flashcards
(29 cards)
State the Rayleigh-Jeans law for energy density.

State the equation for energy density.
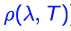
State the equation for heat capacity at lower temperatures according to classical physics.
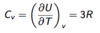
Give the equation for the kinetic energy of electrons in the photoelectric effect.
EK = 1/2 mev2 = hv - Ø
me: mass of electron
v2: velocity (v = c ÷ wavelength)
Ø = hv = hc/i\
h: energy of photon
c = i\v
Give the general form for an eigenvalue equation.
(operator)(eigenfunction) = (eigenvalue)(eigenfunction)
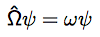
Give the equation for the probability of finding a particle between x1 and x2.
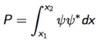
Give the equation for a normalised wavefunction.
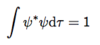
Give the equation for two orthogonal wavefunctions.
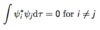
Give the expectation value of an operator (sigma).
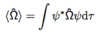
Give the equation for the Heisenberg uncertainty principle.
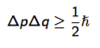
State the Schrödinger equation for a 1-dimensional system.
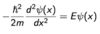

What is h(bar)2 equal to?
h(bar)2 = h2 / 4π2
Sketch the wavefunction and probabilty density fot the n=1 and n=2 states of a particle in a infinite well.

Sketch and annotate the diagram for a partcile at a finite barrier.

Sketch the graph showing the quantum mechanical path of tunneling.

Sketch the ground state wavefunction of a harmonic oscillator.

Sketch the first excited state wavefunction of a harmonic oscillator.

Give the equation for the harmonic oscillator.
V(x) = 1/2 kfx2
Inserting V(x) into the Schrodinger equation gives which allowed energy levels? (2)
Ev = (v + 1/2) h(bar)w
w = (kf/m)1/2
v = 0, 1, 2…
m: mass of particule, for diatomics use mu
w: frequency
Ev: vibrational energy
Sketch a diagram showing that the particle is more likely to go from ground-ground state to excited-excited state.

State Couloumb’s Law.
V = - (Ze2) ÷ (4πeor)
eo: permitivity of free space
Z: nuclear charge
Give all the quantities that are equal to 1 in atomic units.
- mass of electron me
- charge of electron e
- reduced Plank’s constant h(bar) (h/2π)
- Coulomb’s constant 1/4πeo
Write the Schrodinger equation for hydrogen.