Endocrine Flashcards
Be able to give the role (and/or alternate name) of the following a.) thyroxine-binding prealbumin b.) transthyretin c.) thyroglobulin d.) thyrotropin e.) thyrotropin-releasing hormone (TRH) f.) thyroid stimulating hormone
- a.) aka transthyretin (TTR), semi-specific thyroid-binding protein, also binds retinol - b.) aka thyroxine-binding prealbumin, semi-specific thyroid-binding protein, also binds retinol - c.) glycoprotein colloid in thyroid gland (in lumen of follicle) that binds DIT, MIT, T3 and T4, ie. Is reservoir for thyroid hormone - d.) aka TSH, released from anterior pituitary, stimulates synthesis/secretion of thyroid hormone - e.) released from hypothalamus, stimulates release of TSH from anterior pituitary - f.) aka thyrotropin, released from anterior pituitary, stimulates synthesis/secretion of thyroid hormone
Describe the control mechanisms of the HPA (hypothalamus-pituitary-adrenal)-axis.
- CRH released from hypothalamus binds to G-protein receptors on corticotropic cells of anterior pituitary stimulating secretion of ACTH, synthesize of ACTH from POMC. ACTH binds to MC2R (melanocortin 2 receptor) on adrenal cortical cells = synthesize and secretion of cortisol and androgens primarily - Cortisol negatively feeds back on both hypothalamus and pituitary
Identify cells of origin and functions of GnRH, LH, FSH, testosterone, ABP (androgen binding protein) and inhibin B
- GnRH: from hypothalamus, induces release of LH and FSH from anterior pituitary - LH: from ant pituitary, stimulates testosterone production and secretion in Leydig cells - FSH: from ant pituitary, stimulates Sertoli cells to produce ABP, proliferate (growth factors), seminiferous tubule growth, aromatase production (converts T to estradiol), spermiogenesis - Testosterone: from Leydig cells, facilitates spermatogenesis + many other functions (see next objective) - ABP: from Sertoli cells, concentrates testosterone in seminiferous tubules for spermatogenesis - Inhibin B: from Sertoli cells, secreted into blood where it inhibits FSH production from anterior pituitary
Explain the control of secretion and the functions of the pituitary gland. Be able to list the anterior and posterior pituitary hormones and give their functions.
1.) Anterior a.) ACTH (adrenocorticotropic hormone): +CRH, stimulates synthesis and secretion of adrenocortical hormones (mainly cortisol, androgens and aldosterone – no effects on this at physiologic levels), accelerates melanin synthesis b.) TSH: +TRH, stimulates synthesis and secretion of T4 and T3 from thyroid gland c.) FSH: +GnRH, growth of follicles in ovaries and sperm maturation d.) LH: +GnRH, stimulation of testosterone synthesis in testes and stimulation of ovulation, CL formation, E and P synthesis in ovaries e.) GH: +GHRH, +ghrelin, -GHIH, stimulates protein synthesis, overall growth of cells/tissues, production of IGF f.) PRL: +TSH, -PIH, development of female breast and milk production 2.) Posterior a.) ADH: see renal b.) Oxytocin: +breastfeeding & childbirth, causes milk let down, positive feedback loop of contraction of uterus
Describe where vit D precursors are transformed into vit D3 and where vit D3 is converted (2 steps, 2 organs) into the most active form of vit D. What is name for this most active form?
- Vit D in diet is a prohormone that has to undergo two hydroxylation rxns to become active form. Starts in skin where light causes formation of vit D3. D3 is converted in liver to 25-hydroxycholecalciferol. This is converted in kidney to 1, 25-dihydroxy vit D aka calcitriol (most active form) under stimulation by PTH.
Identify the distinguishing features between T1 and T2 DM as they relate to: beta-cell function, insulin sensitivity, blood glucose levels, ketone production.
a.) T1 - beta-cell function: eventual destruction = absolute insulin deficiency - insulin sensitivity: sensitive to insulin, simply lack of - blood glucose levels: hyperglycemia (diet, glucagon stimulation) - ketone production: ketogenesis stimulation under low/no insulin b.) T2 - beta-cell function: no destruction, can be secretory defect, but not absolute lack of insulin - insulin sensitivity: resistance (receptor # change or affinity change) - blood glucose levels: hyperglycemia - ketone production: high insulin inhibits ketogenesis
Describe change in basal body temp during sexual cycle and know hormone responsible for this change.
- Basal body temp changes throughout the sexual cycle, but especially during the time of ovulation where is rises 0.5 – 0.8 degrees. This indicates the ovulation has occurred and the corpus luteum has been formed. Temp change is mediated by progesterone.
Describe male hypothalamus-pituitary-gonadal (HPG)-axis

List and describe stages of the male sexual act
1.) Erection: PSNS, vasodilation of penile arteries 2.) Lubrication: PSNS, secretion of mucus from Cowper’s 3.) Emission: SNS, contraction of ampulla in vas, contraction of seminal vesicle and prostate, contract of internal sphincter of bladder 4.) Ejaculation: spinal reflex, somatic motor, rhythmic contraction of muscles at base of penis and pelvic floor
When suspecting cortisol deficiency, when is the most opportune time to measure?
- In the morning. Normally levels are most highest before and after waking.
Recognize the acute complications potentially experienced by pts with DM and the general therapeutic approach to addressing these complications.
- hypoglycemia: - ANS symptoms: tachycardia, sweating, tremors, nausea, hunger - Neuro: irritability, confusion, HA, speech changes, blurred vision, tiredness, LOC, seizure, coma, death - Therapy: glucose, glucagon 2. DKA: more common with type I than II - Hyperglycemia, acidic blood pH, ketones, polyuria, fatigue, nausea, vomiting, stupor, confusion, coma - Therapy: restore plasma volume, reduce glucose, correct acidosis, replenish electrolytes
Describe the key hormone changes that occur during the transition to pregnancy.
- implantation = hCG secretion from placenta = hCG binds LH receptors on theca-lutein and granulosa-lutein cells = no regression of CL - CL secretes relaxin = inhibition of myometrial contractions - Week 8 = placenta takes over steroid synthesis
Identify and describe the causes, symptoms and pathophysiology of primary adrenal insufficiency (Addison’s dz).
a.) Primary adrenal insufficiency (Addison’s dz) – deficiency in cortisol, aldosterone and androgens - Cause: most commonly = autoimmune, in developing countries = TB - Symptoms: hypoglycemia, hyponatremia, hypotension, weight loss, hyperkalemia, hyperpigmentation (POMC derived melanocyte stimulating hormone increased) - Pathophysiology: destruction of all zones of adrenal cortex, therefore deficiency in cortisol, aldosterone and androgens. ACTH/CRH high d/t loss of –ve feedback. Hypotension (d/t loss of Ne/EPI receptors).
Testosterone diffuses into blood from testes. In what forms is it bioavailable? Not bioavailable?
- Bioavailable when in free/unbound and/or albumin-bound forms - Not bioavailable when in SHBG (sex hormone-binding globulin) forms
Describe biosynthetic pathways for testosterone, dihydrotestosterone (DHT) and estradiol
- Testosterone production occurs in Leydig cells. Conversion of cholesterol into pregnenelone via cholesterol desmolase (aka P450 scc, cholesterol side chain cleavage ez) and then further by 17 alpha hydroxylase, 3 beta hydroxysteroid DH and 17 beta hydroxysteroid DH into testosterone - Testosterone is converted into DHT by 5 alpha-reductase in other tissues (mainly prostate) - Testosterone converted into estradiol by P450 aromatase
Describe impact of steroids on proliferative and secretory changes of the uterine endometrium.
1.) Follicular phase a.) Ovary: I. LH/FSH = estrogen production/secretion. Estrogen exerts negative feedback on anterior pituitary primary. II. Estradiol increases expression of FSH receptors resulting in increased sensitivity of granulosa cell to FSH, rapid growth of follicles occurs. Estradiol and FSH increase expression of LH receptors on theca cells increasing androstenedione secretion. For these reasons that rise of estradiol occurs with very slight rise in LH and FSH. III. One mature / dominant follicle reaches readiness to ovulate. Remaining follicles undergo atresia, may be in part to decline in FSH. IV. Inhibin B secreted from granulosa cells exerts negative feedback at anterior pituitary to decrease FSH secretion b.) Endometrium: I. Estrogen causes proliferation of epithelial cells, stromal cells, growth of endometrial glands, BV development and mucus secretion 2.) Ovulation: - ~36 hours of high estrogen exerts positive feedback at anterior pituitary primarily resulting in LH/FSH surge at day 14. LH surge required. - LH stimulates rupture of follicle (ovulation) by promoting remodeling via changes in gene expression 3.) Luteal phase a.) Ovary: I. Corpus luteum forms from thecal, granulosa, fibroblast, endothelial, immune cells and lipids. Serves as temporary endocrine gland producing both estradiol and progesterone. Reaches mature states around 7-8 days post-ovulation. II. Rise in progesterone and inhibin A exert a negative feedback at ant pituitary. As a result, LH/FSH decline. Steroid production also declines. III. 12 days post ovulation, CL regresses and becomes corpus albicans causing further decline of steroid production. IV. With negative feedback inhibition relieved, LH/FSH production begins to rise again. b.) Endometrium: I. Under progesterone, endometrium undergoes the following changes: increased complexity of vascular and glandular structure, accumulation of substances in glands, deposition of lipids/glycogen in stromal cells, increased blood supply. II. With decline in steroids, release of proteolytic enzymes causes lysis of tissue. Increase of PG production increases myometrial contractions.
Describe the relative significance of obesity, genetics, environment, and the immune system in the development of T1 and T2 DM.
a.) T1: immune-mediated beta-islet destruction (T-lymphocytes, not antibody); genetic predisposition (MHC, polymorphism…), environmental factors can trigger (virus, toxins) b.) T2: obesity (visceral adiposity), secondary to disease (pancreatitis, CF, CA, somatostatinoma), drug-induced, genetic predisposition (high concordance bw monozygotes), environmental (high caloric intake, low physical activity)
List the regulators of glucagon secretion/release
- Stimulators: catecholamines, CCK, gastrin, SNS and PSNS stimulation - Inhibitors: glucose, FFAs
What is hypogonadism? Identify the differences between primary and secondary male hypogonadism.
- Androgen deficiency - Primary (aka hypergonadotropic hypogonadism): d/t testicular dysfunction/lesion as seen in cryptorchidism, Klinefelter syndrome (XXY) where inhibition of testosterone is released and increases in GnRH are seen. - Secondary (aka hypogonadotropic hypogonadism): d/t pituitary dysfunction/lesion where decrease in circulating GnRH leading to low testosterone in normal to low LH/FSH environment. Seen in Kallmann syndrome (inherited malfunction of GnRH neurons).
Describe regulatory signals that control ovulation. Describe formation and decline of the corpus luteum.
- ~36 hours of high estrogen in the follicular phase exerts positive feedback at anterior pituitary primarily resulting in LH/FSH surge at day 14. LH surge required. - LH stimulates rupture of follicle (ovulation) by promoting remodeling via changes in gene expression - Corpus luteum forms from thecal, granulosa, fibroblast, endothelial, immune cells and lipids. Serves as temporary endocrine gland producing both estradiol and progesterone. Reaches mature states around 7-8 days post-ovulation. - Rise in progesterone and inhibin A exert a negative feedback at ant pituitary. As a result, LH/FSH decline. Steroid production also declines. - 12 days post ovulation, CL regresses and becomes corpus albicans causing further decline of steroid production.
Describe functions of thyroid hormones. What are the target cells of the thyroid hormones? Where are thyroid hormone receptors located (on cell surface or intracellular)?
- Functions: normal body growth in early life, stimulates cell metabolism and activity, increase CO directly/indirectly, causes rise in cholesterol in blood, TSH secretion - Target: virtually all tissues - Receptor: nuclei
Describe bone remodeling. Which cell type is responsible for bone deposition? Bone resorption? What are roles and alternate names of OPGL and OPG? Which increases bone resorption? What roles do PTH and vit D play in bone resorption?
- Cell type: osteoblasts deposit bone, osteoclasts resorb bone via indirect PTH stimulation - Roles of OPGL/OPG: PTH binds to receptors on osteoblasts causing them to release cytokines including osteoprotegerin ligand (OPGL aka RANK ligand). OPGL activates receptors on proosteoclast cells causing differentiation into multinucleated osteoclasts = bone resorption. OPG (osteoprotegerin) is a cytokine and decoy receptor for OPGL preventing it from binding proosteoclast and therefore preventing bone resorption - PTH and vit D promote bone resorption
Identify the catecholamines released by the adrenal medulla.
- NE and Epi in response to ACH from pre-ganglionic neurons binding to chromaffin cells in medulla of adrenal gland
List the signs and symptoms, as well as the cause of these, of T1 and T2 DM. Compare and contrast the metabolic changes occurring between two patient groups.
a.) T1: - Polyuria/nocturia (osmotic diuresis) - Polydipsia (hyperosmolar state) - Blurred vision (hyperosmolar state) - Weight loss/polyphagia (acute = depletion, chronic: muscle mass loss) - Weakness/dizziness (hypotension, K loss) - Paresthesias (sensory nerve dysfunction) - Consciousness changes (hyperosmolarity, dehydration, DKA) b.) T2: - Initially asymptomatic: pancreas able to overcome - Infections: skin, vaginitis (abundance of glucose for microorganisms) - Neuropathy: retinopathy, peripheral neuropathy (abundance of glucose) - Classic signs (see above): polyuria, polydipsia, blurred vision, fatigue, weakness - Obesity: VLDL secretion - metabolic syndrome (hyperglycemia with hyperinsulinemia, dyslipidemia and HTN). How? Insulin = Na retention, insulin = VLDL secretion
Describe process of fertilization, including capacitation and the acrosome rxn and the movement of the blastocyst to the uterus.
a.) Sperm capacitation = increased motility and preparation for acrosomal rxn in isthmus of fallopian tube b.) Sperm binds zona pellucida, acrosomal rxn occurs allowing zona pellucida penetration by proteolysis c.) Sperm ovum membranes fuse, release of sperm nucleus into ovum d.) No further sperm able to penetrate e.) Fertilized egg undergoes mitosis = blastocyst f.) Blastocyst invades endometrium
Describe functions of epididymis, vas deferens, seminal vesicle, prostate and bulbourethral gland
1.) epididymis: maturation of sperm, secretes H+ to acidify luminal fluid (maintains immotility of sperm), storage of sperm, re-absorption of aging sperm 2.) vas deferens: storage and transportation of sperm 3.) seminal vesicle: secretion/storage of fructose product, PG, ascorbic acid, fibrinogen/thrombin 4.) prostate: secretion/storage of fluid rich in phosphatase and PSA (protease) 5.) bulbourethral (Cowper’s): mucus secretion upon arousal
Be able to define the following terms and/or describe the process: a.) iodide trapping b.) organification
- a.) Process of concentrating iodide inside epithelial cells of thyroid gland. 2Na-I symporter brings iodide in, moves to apical (luminal) membrane where it crosses into lumen via pendrin transporter. Oxidized to iodine. - b.) Process of binding iodine to tyrosine on thyroglobulin
Describe the physiological role of key pancreatic hormones (GLP-1, IAPP, pancreatic polypeptide, somatostatin, ghrelin) and predict the pharmacological impact of administering analogues or inhibitors of these agents to patients
- GLP-1: secretion in response to glucose, lipids and PSNS; promotes production and secretion of insulin and somatostatin, protects promotes growth of beta-cells. It is degraded by DPP-4. Activators of this, inhibitors of DPP-4 have potential for therapy in diabetic patients. - IAPP (islet amyloid polypeptide, amylin): secretion in response to stimuli regulating insulin (in same vesicle); act to decrease glucagon secretion, inhibit gastric motility/emptying and regulate appetite. Analogues use to tx pts with type I and II DM. - Pancreatic polypeptide: secretion regulated by neuronal control through vagus nerve; regulates exocrine function of pancreas, GB contraction, gastric acid secretion and GI motility - Somatostatin: secretion in response to stimuli regulating insulin secretion; inhibits insulin secretion in paracrine fashion by activating SSTR-5 receptors. Pharmacological analogues don’t adversely affect CHO homeostasis d/t more SSTR receptors. - Ghrelin: pancreatic function not well understood; shown to induce gastric emptying, promote gastric acid secretion and NB = increase appetite.
List the effects of testosterone, dihydrotestosterone (DHT) and estradiol
1.) Testosterone: spermatogenesis (via conversion into estradiol), internal genitalia development, increased skeletal muscle mass and strength, erythropoiesis, increased bone strength, increased resting metabolic rate, inhibits lipid accumulation, stimulates lipolysis, inhibits adipocyte precursor differentiation 2.) DHT: external genitalia development, increased hair follicle growth 3.) Estradiol: epiphyseal closure and increased bone density, libido
Diagram the fluctuations in hormones (LH, FSH, Estrogen, Progesterone, Inhibin) during the female sexual cycle and how these changes correlate with endometrial changes.
.
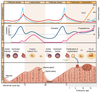
List cell types found in pancreatic islets and their specific hormone secretions from each cell type
1.) alpha: glucagon, proglucagon 2.) beta: insulin, proinsulin, C-peptide, islet amyloid polypeptide (IAPP), GABA 3.) delta: somatostatin 4.) epsilon: ghrelin 5.) PP/F cells: pancreatic polypeptide
What are the causes and symptoms of hyperparathyroidism, hypoparathyroidism and pseudohypoparathyroidism.
1.) Hyperparathyroidism (primary): high PTH - Cause: benign adenoma, parathyroid cancer - Signs, symptoms: hypercalcemia, hypophosphatemia, asymptomatic, formation of kidney stones, weak bones/fractures d/t resorption, constipation / polyuria d/t hypercalcemia, enlargement of parathyroid glands 2.) Hypoparathyroidism: low or no PTH - Cause: parathyroidectomy secondary to thyroid surgery - Signs, symptoms: steady decline in plasma calcium, neuromuscular excitability leading to hypocalcemic tetany (potential closer to threshold) 3.) Pseudohypoparathyroidism: normal or elevated PTH - Cause: problems with PTH receptor resulting in decrease tissue response to PTH - Signs, symptoms: see hypoparathyroidism
Defect of 3beta-hydroxysteroid DH would mean what in terms of adrenal hormone synthesis?
- Failure to synthesize aldosterone and cortisol, only ability to produce DHEA
Define the cellular consequences that occur following activation of the insulin receptor
- Insulin binding insulin receptor activates intracellular cascade leading to: 1.) Translocation of GLUT4 receptors into plasma membrane for uptake of glucose in plasma 2.) Mitogenesis 3.) Increases in protein synthesis 4.) Increases in glycogen synthesis
Be able to state if T4, TSH and TRH increase or decrease in pts with the following conditions: a.) primary hypothyroidism b.) pituitary hypothyroidism (aka secondary hypothyroidism) c.) hypothalamic hypothyroidism (aka tertiary hypothyroidism) d.) pituitary hyperthyroidism (aka secondary hypothyroidism) e.) Graves’ disease
- a.) primary hypothyroidism: low T4, high TSH, high TRH - b.) pituitary hypothyroidism (aka secondary): low T4, low TSH, high TSH - c.) hypothalamic hypothyroidism (aka tertiary): low T4, low T4, low TRH - d.) pituitary hyperthyroidism (aka secondary): high T4, high TSH, low TRH - e.) Grave’s dz: high T4, low TSH, low TRH
List the plasma forms of calcium. Which forms are filtered in glomerulus? In which form is ~50% of the total calcium?
Three forms: 1.) Free / ionized (~50% of total): alkaline = more bound to protein, acidic = less bound to protein 2.) Protein-bound (~40%) 3.) Bound to small diffusible anions (~10%) - Non-protein forms are filtered in glomerulus?
Describe the different types of diabetes insipidus.
1.) Neurogenic: genetic defect or trauma/infection/CA that interferes with production/release of ADH. Collecting ducts cannot produce concentrated urine, patients produce a large amount of dilute urine. 2.) Nephrogenic: defect in kidney (potentially mutation in V2 receptor) where ADH doesn’t have effect or where production/insertion of aquaporins doesn’t occur in order to produce concentrated urine. 3.) Acquired diabetes insipidus: acquired neurogenic as above or pt on lithium 4.) Psychogenic polydipsia leading to diabetes insipidus: pt should refrain from water
Compare and contrast the impact of glucagon and insulin on the metabolic processes of gluconeogenesis, glycogenesis, glycogenolysis, glycolysis, and ketogenesis
a.) Gluconeogenesis: +glucagon, -insulin b.) Glycogenesis: +insulin c.) Glycogenolysis: +glucagon d.) Glycolysis: +insulin, -glucagon e.) Ketogenesis: - TGL to FFAs: +low insulin, +glucagon, -high insulin? - AAs to OAA to glucose: +low insulin, -high insulin?

