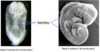
Early fetal development Flashcards
LO:
- Embryo development: Summarise the key developmental events occurring in embryo in the first trimester.
- Pregnancy physiology: Summarise the key changes in maternal physiology across the course of pregnancy
Part 1: Pre- and Peri-implantation development
Measuring time in embryo-fetal development-ages
If we want to track the chronology of embryo development, we need a measure of embryo foetal developmental time. Perhaps the most straightforward of these is fertilisation age. this is usually expressed in days post fertilisation or weeks post fertilisation. Difficult to know exactly as variability in time between intercourse and fertilisation occuring. However, we can infer it if we know the time of ovulation, becasue generally fertilisation has to have occured within 24hrs of the ovulation occuring. But on the whole, although fertilisation age is a useful measure, and is the one that we’ll use for most of this session, in practical terms it’s not particularly useful.
We therefore frequently use the gestational age, and this is calculated from the start of the last menstrual period. So the menstrual cycle is 28 days long, ovulation occurs halfway through this, and if that ovulated oocyte is then fertilised, implants into the endometrium, it will signal back to the corpus luteum to produce progesterone, and that will rescue the endometrial lining. So we will then miss the period and won’t see menstrual bleeding in this part of the cycle. If that happens, we can then infer that ovulation and fertilisation must have happened in this particular menstrual cycle, and so then we use the gestational age calculation from day 0 essentially of this particular menstrual cycle. Consequently, gestational age is always 14 days longer than fertilisation age, because it goes from the start of the menstrual period, rather than the point of ovulation, or the point of fertilsation. We can estimate gestational age quite simply from the pattern of a woman’s menstrual periods, or we can undertake an early obstetric ultrasound and compare the size of the embryo to reference size charts.
A further way of tracking embrological development, is the Carnegie staging system and this utilises a collection of embryos at the Carnegie institute in Washington. The important thing about the Carnegie staging is that it is based on embryo features rather than time. And because it’s based on structure and development of the embryo, the presence or absence of particular features, it allows us to directly compare developmental rates and events between species. The Carnegie stage in humans, covers the window of roughly 0-so the time of fertilisation, through to about 60 days post fertilisation.

Measuring time in embryo-fetal development-stages
We can also divide pregnancy up into different sections corresponding to periods of embryological development.
First stage is embryogenic stage which runs from point of fertilisation to about 14 to 16 days post fertilisation. In this stage, we essentially form the early embryo from the fertilised oocyte. This stage is characterised by the formation of 2 cell types, the pluripotent embryonic cells, and these will go forward and contribute to the organs of the foetus, and the extra embryonic cells which contribute to the support structures such as the placenta.
Following the embryogenic stage, we have the embryonic stage and that runs from about 16 days post fertilisation to about 50 days post fertilisation. This stage is characterised by the establishment of the germ layers and the differentiation of tissues and the establishment of the body plan more akin to an adult organism.
Embryogenic and embryonic stages comprise the first trimester or first 1/3, roughly 12 weeks of pregnancy and the foetal stage corresponds to the second and third trimester, (second 12 week block and final 12 week block of pregnancy). And so the transition from embryo to foetus occurs roughly at the end of the first trimester.
Once that body plan is established and the major organ systems are specified, we then move into the foetal stage. And so the organ systems are present, although some of those organ systems may not be in the place that they will be at birth. So the foetal stage can be characterised by migration of some organ systems to their final location. We also see extensive growth and acquisition of foetal viability, which is the ability of the foetus to survive outside the womb.

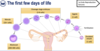
The first few days of life
Start off with an ovulated oocyte here which is a single cell. And that’s going to go through a process of fertilisation, where it will meet a sperm, and when it does so, it becomes a zygote. The zygote then undergoes a series of mitotic divisions known as cleavage divisions. That gives us a 2 cell embryo, a four cell embryo and an eight cell embryo. And these 2, 4 and 8 cell embryos, are known as cleavage stage embryos. The 8 cell embryo proceeds with further mitotic divisions, giving us the Morula at the 16 cell stage. And finally the morula progresses to form a blastocyst, which is comprised of 200-300 cells.
This developmental trajectory is happening as the oocyte and early embryo is migrating along the fallopian tube and into the uterus where it can implant. It’s also important to note that the zona pellucida, the protein shell that surrounds the oocyte at ovulation, is present for all of these stages, so all of these cell divsions are occuring within the constriction of the zona pellucida.
Maternal-to-Zygotic transition
The first major developmental event in the embryo is the Maternal-to-Zygotic transition which occurs at the 4 to 8 cell stage.
Now up until about the 4 to 8 cell stage, none of the genes of the embryo are transcribed. Instead the development of the embryo, and the divisions of the embryo is dependent on maternal mRNAs and proteins. And these maternal mRNAs and proteins are stored during the process of oocyte development, so before ovulation.
At the Maternal-to-Zygotic transition, what happens is the embryonic genes take over, so we start to get transcription from embryonic genes, and we lose the reliance on the maternal mRNAs and proteins. The embryo itself starts to make some increased amounts of protein, and we see maturation of some of the organelles, particularly the mitochondria and the golgi involved in metabolism and protein synthesis and distribution.

Compaction starts the formation of the first two cell types
The second major event is compaction. This happens around the 8 cell stage, and embryo compaction gives us our first 2 cell lineages. As the cells are dividing by mitosis, they’re initially all spherical and radially symmetrical, but as we go through a series of division, some of the cells on the outside start pressing up against the zona pellucida. This basically causes a developmental change in these cells on the outside of the embryo, and they move from being spherical, to being wedge shaped. What then happens is tight junctions and desmosomes start to form between these cells on the outside. And when these cells become tightly bound to one another, it creates a barrier to diffusion from the outside of the embryo to the cells that are in the middle. The outer cells also become polarised, with a distinct apical and basal polarity.
So this process of compaction, and we call it compaction, because it’s those outer cells binding to one another and pulling in, that essentially creates 2 distinct cell types in the early embryo. An inner cell population, given here in pink, which is shielded from the outside, and this tightly bound outer cell population here in green. And these 2 cell types are going to develop further with the outer cells forming this shell of the blastocyst here and the inner cells forming this little clump at one end of the blastocyst.

Looking at the blastocyst in more detail:
Blastocyst formation establishes two cell types
Whole thing is contained in zona pellucida. Purpose is to protect the embryo, but at earlier stages also to prevent multiple sperm fertilising the oocyte.
We now have our 2 cell populations. We have the inner cell mass, so this was this inner cell population. And these cells will give rise to the pluripotent embryonic cells that will contribute to the final organism.
On the outside we have what’s called the trophectoderm, so these were those outer cells and the trophectoderm lineage will give rise to the extraembryonic cells that make up the extra embryonic support structures such as the placenta.
We also see at the blastocyst stage, the formation of this fluid filled cavity called the blastocoel, and this occurs because the trophectoderm cells pump sodium ions into the centre of the embryo and water then follows this osmotically, to create this large fluid filled space in the middle.

Now once the embryo reaches this stage, its developmental potential becomes limited, because it’s still retained within the zona. So in order for this embryo to progress further it needs to undergo a procedure called hatching.

Hatching really is the escape of the blastocyst from the zona pellucida shell and it’s achieved through a combination of enzymatic digestion, so the blastocyst secretes enzymes and a number of cellular contractions of the embryo, which together weaken a point of the zona pellucida, which allows the blastocyst to extrude itself out of the zona shell. So when it does that, it can then go ahead and implant. If the blastocyst doesn’t escape the zona shell, it can’t undergo implantation into the endometrium.

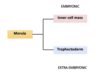
Separation of embryonic cell lineages I (at the morula-blastocyst stage)
We get the formation of the inner cell mass which is going to contribute to the embryonic tissues, and the trophectoderm, which is going to contribute to the extra embryonic tissues.
Peri-implantation events (~day 7-9 post fertilisation)
Once the embryo has undergone it’s initial connection with the endometrium, we get a couple of differentiation events where the cell types that we’ve previously mentioned undergo some changes.
Firstly the trophectoderm lineage separates into the syncitiotrophoblast and the cytotrophoblast. The syncitiotrophoblast is invasive, it invades the uterine endometrium and as it does so it starts to degrade the cells of the endometrium and ultimately breaks down capillaries which allow those syncitiotrophoblast cells to be bathed in maternal blood. The cytotrophoblasts continue to divide, to add cells to the syncitiotrophoblast.
We also see differentiation of the inner cell mass, so we get 2 populations of cells forming from the inner cell mass. Firstly the epiblast and it’s the epiblast from which foetal tissues and organs will be derived. And also we see a population of cells which lines the underside of the epiblast, facing into the blastocyst cavity., and this is called the hypoblast, and ultimately this will form a structure known as the yolk sac, which is an extraembryonic structure and is important in gut development and early haematopoeisis.

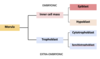
Separation of embryonic cell lineages II
So we can separate our lineages again:
Trophoblast or trophectoderm
Bi-laminar embryonic disc formation (day 12+ of fertilisation)
In this stage the syncitiotrophoblast has continued to expand into the endometrium, but we see particular changes occuring within the epiblast and the hypoblast.
What happens is, some of the epiblast cells become separated from the main block by the formation of this new cavity called the amniotic cavity. So these epiblast cells along the top are going to give rise to the amnion which is one of the extra embryonic membranes.
The epiblast that is left here (below amniotic cavity) is the epiblast which is going to give rise to the foetal structures and organs.
The hypoblast also remains sitting under the epiblast here, so what we end up with is this 2 layer disc of epiblast on the top and hypoblast on the bottom. It’s important to remember this is a cross section through the embryo so the whole thing is spherical, so this actually looks like a disk, like a 2 pence piece (epiblast) on top of a 10pence piece (hypoblast). Called bilaminar disk together. Once embryo reaches bi-laminar disc stage, it’s ready for gastrulation.
the syncitiotrophoblast at this point is also starting to produce human chorionic gonoadotrophin. And it’s detection of the beta subunit of hCG in the maternal blood in the urine, which underpins modern pregnancy testing.

Separation of embryonic cell lineages III
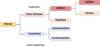
- Pre and peri-implantation development session review:
- Embryo-fetal developmental progression can be measured in different ways.
- Early events separate the embryo into embryonic (inner cell mass) and extra-embryonic cells (trophectoderm)
- Extra-embryonic cells differentiate further into syncitiotrophoblasts and cytotrophoblasts
- Inner cell mass forms bilaminar disc
Part 2: Gastrulation
Video transcript: https://www.youtube.com/watch?v=ADlYn0ImTNg&feature=emb_imp_woyt

Key points to learn:
- Day 15-primitive streak forms along midline in epiblast at caudal end of bilaminar disk
- At cranial end of the embryonic disc the primitive streak expands to create a primitive node which contains primitive pit. (think SNP G)
- This depression continues along the midline of the epiblast towards the caudal end of the streak, forming a primitive groove.
- Once formed cells of the epiblast migrate inwards towards the streak, detach from the epiblast and slip beneath it into the interior of the embryo (invagination).
- The first cells to invaginate through the primitive groove invade the hypoblast and displace it cells, forming proximal layer-the definitive endoderm.
- By day 16, the majority of the hypoblast has been replaced. The remaining cells of the epiblast=ectoderm (most exterior distal layer).
- Some of the invaginated epiblast cells remain in the space between the ectoderm and newly formed definitive endoderm=mesoderm.
- Once the formation of the definitive endoderm and mesoderm are complete, epiblast cells no longer migrate towards the primitive streak.
- Throughout gastrulation the ectoderm continues to form, from the cranial to the caudal end of the embryo, establishing three distinct primary germ layers throughout the whole embryonic disc. The gastrulation process is finally complete.
The Process of Gastrulation
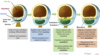
By the end of the second week of development the bi laminar embryonic disc consisting of the hypoblast and epiblast has formed. Throughout the third week of development, this bilaminar disk differentiates to establish three primary germ layers, in a process known as gastrulation.
- Around 15 days after fertilization, a thickened structure forms along the midline in the epiblast near the caudal end of the BI laminar embryonic disc. This is called the primitive streak.
- At this stage the formation of the primitive streak defines the major body axes of the embryo including the cranial end towards the head and caudal ends towards the tail as well as the left and right sides of the embryo.
- At the cranial end of the embryonic disc the primitive streak expands to create a primitive node which contains a circular depression known as a primitive pit. (think SNP G)
- This depression continues along the midline of the epiblast towards the caudal end of the streak, forming a primitive groove.
- Once formed cells of the epiblast migrate inwards towards the streak, detach from the epiblast and slip beneath it into the interior of the embryo. This process is known as invagination. The first cells to invaginate through the primitive groove invade the hypoblast and displace it cells.
- The hypoblast cells are eventually completely replaced by a new proximal cell layer which is referred to as the definitive endoderm.
- By day 16, the majority of the hypoblast has been replaced. The remaining cells of the epiblast, are now referred to as the ectoderm and forms the most exterior distal layer.
- Some of the invaginated epiblast cells remain in the space between the ectoderm and newly formed definitive endoderm. These cells form a germ layer, known as the mesoderm.
- Once the formation of the definitive endoderm and mesoderm are complete, epiblast cells no longer migrate towards the primitive streak.
- Throughout gastrulation the ectoderm continues to form, from the cranial to the caudal end of the embryo, establishing three distinct primary germ layers throughout the whole embryonic disc. The gastrulation process is finally complete.


Gatrulation Session review:
- Formation of the primitive streak defines head-tail and left-right axes of embryo
- Invagination of epiblast cells from that bilaminar disc, down through that pit into the primitive streak forms 3 germ layers:
- Endoderm cells (first through the streak)
- Ectoderm remains on the upper (ventral) surface (and don’t migrate through the primitive streak)
- Mesoderm- do migrate through the primitive streak, but become sandwiched between endo and ectoderm
Primitive streak-primitive node-primitive pit

Part 3: Establishing the body plan
In this final part, we explore how the three germ layers established during gastrulation are fashioned into the organ systems and tissues of the body.
Separation of embryonic cell lineages IV
3 germ layers derived from epiblast at the bilaminar disc stage.

Relationship of organs to germ layers
Endoderm gives rise to GI tract, liver etc. (first column)
Ectoderm gives rise to CNS, skin epithelia etc. (second column)
Mesoderm gives rise to blood compartment, both blood cells and endothelial cells, msuculature, etc. (third column)

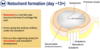
Notochord formation (day ~13+)
The first major event after gastrulation is the normation of the notochord. And this happens around embryonic day 13.
If forms from the primitive streak, and grows forward towards the head end of the embryo. It forms underneath the ectoderm, so we have our 3 germ layers, here is our embryo, we have our primitive streak and the primitive node here, and at the bottom we have the endoderm here in yellow. The mesoderm is sandwiched here in white, and the ectoderm in this orange colour. The notochord forms from edge of primitive streak, growing forward, and it forms underneath the ectoderm, so it’s this rod of cartilage like cells growing forward under the ectoderm. And it grows along the embryo midline, so again we’re defining the head tail and right left axis of the embryo, both by the primitive streak and the formation of the notochord.
Notochord is particularly important, as it acts as a key organising centre for embryo development. It releases a number of growth factor signals, which are particularly important for neurulation, which is the process of central nervous system formation, and also for organising mesoderm development, particularly into the musculature.
The way the notochord organises the development of the neural system is through controlling something called the neural plate. So the neural plate, given here in grey, is an area of ectoderm that sits on top of the embryo. It’s an area of thickened embryo, so they’ll be cell proliferation here, and signals from the notochord beneath the ectoderm are going to move up through the embryo and direct the neural plate to form the neural tube.
Neurulation: forming the neural tube and CNS
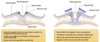
Left image
Here we’re around day 14, 15 of embryo development, and we’ve now sliced through the embryo and we’re looking inside. So we have neural plate here on top of the embryo, the ectoderm here in pale grey, the mesoderm and some endoderm at the bottom.
In order to form the CNS, the notochord sends signals to ths neural plate, to do 2 things. Firstly for part of it to invaginate, and move down towards the notochord, creating the neural groove. And also for 2 areas of the neural plate to move up, creating these 2 crests, called the neural fold. And these are ridges which run along the cranial-caudal axis of the embryo. Within these neural folds, we’re going to get cells called the neural crest cells being specified, and they’re going to migrate away and populate a variety of different tissues.
Right
A couple of days later, as development progresses, the 2 neural folds move towards one another, so they move up and across. And the effect is that the 2 neural folds will eventually meet in the middle and fuse. Because the neural groove has formed below it, the fusion of the 2 neural folds will create a sealed lid essentially over the top of the neural groove, and therefore create a hollow tube, isolated from the surface (as will be covered over) called the neural tube. As the neural tube forms, these migratory neural crest cells, will migrate away and populate various tissues, differentiating as they go.
Importantly though, its the notochord that acts as the major signalling organiser for controlling the process of neurulaton.
Neurulation: forming the neural tube and CNS
Looking a few days later:
Looking a few days later, we can now see that the neural tube has sealed completely, so it has a lumen, surrounded by the cells of the neural plate ectoderm, and the whole area of the top of the neural tube becomes covered in epidermis, which is also ectoderm derived.
As it stands, the neural tube is just a hollow tube that runs the length of the embryo, and therefore is open at the head end and open at the tail end. So for CNS development to proceed, the neural tube needs to be closed both at the head end and the tail end. Closure of the neural tube at the head end occurs a little earlier, about 3 to 4 days earlier than closure at the tail end. But importantly the closure of the neural tube at the head end, is vital for brain structures to subsequently develop.
The failure of the neural tube to close, so failure of the neural folds to completely fuse completely along the whole length of the axis of the embryo, is a relatively common developmental defect. Spina Bifida is an open neural tube at birth, and that’s usually in the area of the lower spine, so that would be more a failure of closure of the tail end of the neural tube. And that’s fairly common, it also comes in various types of varying serverity.
Much more drastic is complete failure of closure at the head end, which leads to anencephaly. And in anencephaly, we have absence of pretty much all of the skull and almost all of the brain. And that arises from a failure to close the neural tube at the head end.
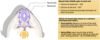
Neural crest cells and their derivatives
Like the rest of the neural tube and the CNS, the neural crest cells are ectoderm derived, they’re also highly plastic and highly migratory during development. We saw they were being specified in the neural folds, and you can see them migrating here throughout the embryo, to give rise to a variety of different cell types.
We can classify the neural crest cells according to where they end up in the embryo. So the population of the neural crest cells migrates to the developing skull and will help contribute to things like middle ear bones and cranial neurones and some facial cartilage.
There is a population of the neural crest cells that migrates to the developing heart.
Neural crest cell that remain in the trunk of the developing embryo, will give rise to dorsal root ganglia, sympathetic ganglia, contribute to the adrenal medulla, and also the pigmentation cells-melanocytes of the embryo.
Defects of neural crest cell migration or specification ie failure to form or migrate normally during embryonic development, leads to a diverse range of birth defects, which can range from pigemntation disorders, through deafness, cardiac and facial weakness, and even failure to innervate the gut.
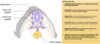
The next key developmental stage after neurulation and neural tube formation is somitiogenesis.
Somitogenesis: segmentation of the body axis
Somitogenesis creates the formation of blocks of mesoderm along the axis of the embryo known as somites.
So here we have cross section through embryo again, and either side of the neural tube and just above the notochord, in red we have almost symmetrical blocks of mesoderm, either side of the neural tube called paraxial mesoderm. As development progresses, blocks of this paraxial mesoderm bud off and there is synchonised budding from both sides at the same time, such that pairs of blocks of mesoderm bud off from this paraxial mesoderm. These pairs of condensed mesoderm are known as somites.
The formation of these somites starts at the head end of the embryo, and works down towards the tail end of the embryo. And the rate at which these somites bud off the paraxial mesoderm, appears to be species specific, as does the number of pairs of blocks that are formed. So in humans, after the first somite pair has formed, we create a new pair of somites, roughly every 90 mins, and leads ultimately to the formation of about 44 pairs of somites in the embryo.