drugs Flashcards
<p></p>
<p>Methotrexate</p>
<p></p>
<p></p>
<p></p>
<ul>
<li>Antimetabolite</li>
<li>AntifolateChemotherapy (Folic acid analog)</li>
<li>Cytotoxic Agent (target all dividing cells)</li>
</ul>
<p></p>
<p></p>

<p></p>
<p></p>
<p></p>
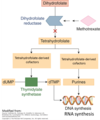
<p>Mechanism of action of methotrexate: What is the primary target?</p>
<p></p>
<p></p>
<p></p>
<p>Dihydrofolate reductase (DHFR) </p>

<p></p>
<p></p>
<p></p>
<p>Mechanism of action of methotrexate</p>
<p></p>
<p></p>
<p></p>
<p>Reduces synthesis of purines
Reduces synthesis of dTMP by inhibiting the necessary cofactor for thymidylate synthetase
Reduces cellular proliferation and induces cellular death by preventing synthesis of RNA and DNA
</p>
<p><p><p><p>Methotrexate therapeutic uses </p></p></p></p>
<p><p><p><p>Useful as single agent in treating acute lymphoblastic leukemia in children
Osteosarcomas often treated with high doses.
Choriocarcinoma (cancer in women’s womb that often originates from placental precursor cells)
Cure rate is 75-90 % with sequential treatments with methotrexate and dactinomycin.
Part of combination therapy for some types of lymphomas, leukemias, and cancers of the breast, head and neck, ovary, and bladder.
</p></p></p></p>
<p><p><p><p>Methotrexate therapeutic uses </p></p></p></p>
<p><p><p><p>Useful as single agent in treating acute lymphoblastic leukemia in children
Osteosarcomas often treated with high doses.
Choriocarcinoma (cancer in women’s womb that often originates from placental precursor cells)
Cure rate is 75-90 % with sequential treatments with methotrexate and dactinomycin.
Part of combination therapy for some types of lymphomas, leukemias, and cancers of the breast, head and neck, ovary, and bladder.
</p></p></p></p>
<p></p>
<p></p>
<p></p>
<p>How do you reduce Methotrexate toxicity?</p>
<p></p>
<p></p>
<p></p>
<ul>
<li>Leucovorin rescue</li>
<li>Leucovorin is administered after and otherwise lethal dose of methotrexate is administered.</li>
</ul>

<p><p><p><p>Methotrexate Resistance</p>
| </p></p></p>
<p><p><p><ul>
<li>Impaired transport</li>
<li>Altered forms of DHFR with decreased affinity for methotrexate</li>
<li>Elevated DHFR expression (gene amplification)</li>
<li>Numerous other mechanisms</li>
</ul>
</p></p></p>
<p><p><p><p>Methotrexate Toxicities</p>
| </p></p></p>
<p><p><p><p>Nephrotoxicity</p>
| </p></p></p>
<p><p><p><p>5-fluorouracil (5-FU) (4 things)</p>
| </p></p></p>
<p><p><p><ul> <li>inhibits thymidylate synthase</li> <li>Pyrimidine Analog</li> <li>Antimetabolite</li> <li>Cytotoxi Agent</li> </ul> </p></p></p>
<p></p>
<p></p>
<p></p>
<p>Mechanism of action of 5-fluorouracil (5-FU)</p>
<p></p>
<p></p>
<p></p>
<ul>
<li>5-FU is metabolized into 5-fluorodeoxyuridine monophosphate (FdUMP), which inhibits thymidylate synthase.</li>
<li>Causes DNA damage by decreasing thymidylate (dTMP) levels, leading to cell death</li>
</ul>

<p><p><p><p>Capecitabine (4 things)</p>
| </p></p></p>
<p><p><p><ul> <li>Antimetabolite</li> <li>Pyrimidine analog</li> <li>is a prodrug of 5-FU that had improved oral bioavailability allowing it to be given orally.</li> <li>Cytotoxic Agent</li> </ul> </p></p></p>
<p><p><p><p>Resistance to 5-FU can be associated with </p></p></p></p>
<p><p><p><p>amplification of thymidylate synthetase.
| </p></p></p></p>
<p><p><p><p>5-FU used as a component of </p></p></p></p>
<p><p><p><p>chemotherapy regimens for breast cancer, head and neck cancers, colorectal, and gastrointestinal.
</p></p></p></p>
<p><p><p><p>5-FU Rarely used as</p></p></p></p>
<p><p><p><p>a single agent
| </p></p></p></p>
<p><p><p><p>5-FU Adverse effects </p></p></p></p>
<p><p><p><p>include oral and GI ulcers and bone marrow suppression
</p></p></p></p>
<p><p><p><p>Capecitabine (3 things)</p></p></p></p>
<p><p><p><p>Antimetabolite
Pyrimidine analog
is a prodrug of 5-FU that had improved oral bioavailability allowing it to be given orally.
</p></p></p></p>
<p><p><p><p>Most important antimetabolite to treat acute myelogenous leukemia (AML)</p>
</p></p></p>
<p><p><p><ul>
<li>Cytarabine (cytosine arabinoside, ara-C)</li>
<li>Only used in treatment of hematologic malignancies</li>
<li>Ara-C is an analog of cytosine.</li>
</ul>
</p></p></p>
<p></p>
<p></p>
<p></p>
<p>Ara-CTP incorporation into DNA inhibits </p>
<p></p>
<p></p>
<p></p>
<p>DNA polymerase, thus halting elongation of DNA molecules</p>

<p></p>
<p></p>
<p></p>
<p>Cytarabine (cytosine arabinoside, ara-C) Only active in </p>
<p></p>
<p></p>
<p></p>
<p>S-Phase</p>

<p><p><p><p>Cytidine deaminase inactivates </p></p></p></p>
<p><p><p><p>ara-C.
| </p></p></p></p>
<p><p><p><p>Cytarabine clinical toxicities</p>
| </p></p></p>
<p><p><p><ul>
<li>Cytidine deaminase levels are quite low in the central nervous system. CNS exposed to higher concentrations than the rest of the body.
<ul>
<li>Can cause cerebellar syndrome Dysarthria (motor speech disorder)</li>
</ul>
</li>
<li>Toxicity is strongly correlated with renal dysfunction, hepatic dysfunction, and advancing age.<br></br>
</li>
</ul>
</p></p></p>
<p></p>
<p></p>
<p>Gemcitabine resistance
| </p>
<p></p>
<p></p>
<p>Reduced activity of deoxycytidine kinase
Tumors increasing production of deoxycytidine
</p>

<p><p><p><p>Cytarabine (3)</p>
| </p></p></p>
<p><p><p><ul> <li>Antimetabolite</li> <li>Pyrimidine analog</li> <li>Cytotoxic Agent</li> </ul> </p></p></p>
<p></p>
<p></p>
<p></p>
<p>Gemcitabine(3)</p>
<p></p><p></p><p></p><ul> <li>Antimetabolite</li> <li>Pyrimidine analog</li> <li>Cytotoxic agent</li> </ul>
<p></p>
<p></p>
<p></p>
<p>Gemcitabine Cytotoxic effects</p>
<p></p>
<p></p>
<p></p>
<ul>
<li>Incorporated into DNA, which inhibits synthesis and function</li>
<li>Inhibits ribonucleotide reductase (reduces pools of dNTPs, which are necessary for DNA synthesis</li>
</ul>

<p><p><p><p>Gemcitabine used in treatment of </p></p></p></p>
<p><p><p><p>a wide range of cancers including: pancreatic, non–small cell lung, ovarian, bladder, etc…
</p></p></p></p>
<p><p><p><p>Gemcitabine resistance</p>
| </p></p></p>
<p><p><p><p>Reduced activity of deoxycytidine kinase Tumors increasing production of deoxycytidine</p>
</p></p></p>
<p></p>
<p></p>
<p>6-Thioguanine (6-TG) (4)</p>
<p></p><p></p><ul> <li>Cytotoxic agent</li> <li>Antimetabolite</li> <li>Purine analog</li> <li>treatment of acute lymphoblastic leukemis.</li> </ul>
<p></p>
<p></p>
<p>6-Mercaptopurine (6-MP) (4)</p>
<p></p><p></p><ul> <li>Cytotoxic agent</li> <li>antimetabolite</li> <li>Purine analog</li> <li>Treatment of acute lymphoblastic leukemia</li> </ul>

<p></p>
<p></p>
<p>6-Thioguanine (6-TG) 6-Mercaptopurine (6-MP)</p>
<p></p>
<p></p>
<ul>
<li>6-MP was the first purine analog used in cancer chemotherapy</li>
<li>Treatment of acute lymphoblastic leukemia (ALL)</li>
<li>Largely replaced by newer antipurines fludarabine and cladribine</li>
</ul>
<p></p>
<p></p>
<p>Mechanism of action of 6-TG (6-Thioguanine)</p>
<p></p>
<p></p>
<ul>
<li>activated to thio-GMP and thio-IMP hypoxanthine-guanine phospho- ribosyl transferase (HGPRT).</li>
<li>Decreased activity of HGPRT common mechanism of resistance.</li>
<li>Thiopurine methyltransferase (TPMT) inactivates 6-MP.</li>
<li>Common gene variant (polymorphism) causes reduced TPMT activity.
<ul>
<li>Can lead to life-threatening toxicity</li>
</ul>
</li>
</ul>

<p></p>
<p></p>
<p>Mechanism of action of 6-Mercaptopurine (6-MP)</p>
<p></p>
<p></p>
<ul>
<li>activated to thio-GMP and thio-IMP hypoxanthine-guanine phospho- ribosyl transferase (HGPRT).</li>
<li>Decreased activity of HGPRT common mechanism of resistance.</li>
<li>Thiopurine methyltransferase (TPMT) inactivates 6-MP.</li>
<li>Common gene variant (polymorphism) causes reduced TPMT activity.
<ul>
<li>Can lead to life-threatening toxicity</li>
</ul>
</li>
</ul>

<p></p>
<p></p>
<p>Fludarabine (4)</p>
<p></p><p></p><ul> <li>Cytotoxic agent</li> <li>antimetabolite</li> <li>newer purine analog</li> <li>Commonly used to treat chronic lymphocytic leukemia (CLL) by itself or in combination with cyclophosphamide and rituximab.</li> </ul>

<p></p>
<p></p>
<p>Fludarabine Mechanism</p>
<p></p>
<p></p>
<ul>
<li>Deoxycytidine kinase activates drug in cells by converting it to the tri-phosphate form.</li>
<li>Incorporated into DNA and RNA</li>
<li>Inhibits DNA polymerase and ribonucleotide reductase (RNR)</li>
<li>Inhibits RNA function, including mRNA translation into proteins</li>
</ul>

<p><p><p>Fludarabine resistance</p></p></p>
<p><p><p>commonly caused be decreased activity of deoxycytidine kinase and drug efflux.
</p></p></p>
<p></p>
<p></p>
<p>Cladribine (4)</p>
<p></p><p></p><ul> <li>Cytotoxic Agent</li> <li>Newer purine analog</li> <li>antimetabolite</li> <li>Standard therapy for hairy cell leukemia (HCL) (curative intent) "when your HAIR gets wet it DRIPS"--> claDRIBine</li> </ul>

<p></p>
<p></p>
<p>Cladribine Mechanism</p>
<p></p>
<p></p>
<ul>
<li>Deoxycytidine kinase activates drug in cells by converting it to the tri-phosphate form.</li>
<li>Incorporated into DNA</li>
<li>Causes strand breaks</li>
<li>Potent inhibitor of ribonucleotide reductase (RNR)</li>
</ul>
<p></p>
<p></p>
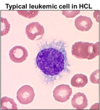
<p>Cladribine resistance</p>
<p></p>
<p></p>
<p>commonly is associated with decreased activity of deoxycytidine kinase, drug efflux, and increased RNR expression</p>
<p><p><p>Methotrexate Mechanism</p>
| </p></p>
<p><p><p>Inhibits dihydrofolate reductase, which reduces precursors for RNA and DNA synthesis</p>
</p></p>
<p><p><p>5-fluorouracil (5-FU)
| Mechanism</p></p></p>
<p><p><p>Incorporated into DNA and RNA, which inhibits synthesis and function
Inhibits thymidylate synthetase, which reduces DNA precursors
</p></p></p>
<p><p><p>Cytarabine (ara-C)
| mechanism</p></p></p>
<p><p><p>Incorporated into DNA and RNA, which inhibits synthesis and function
Inhibits DNA synthesis by inhibiting DNA polymerase
</p></p></p>
<p><p><p>Gemcitabine Mechanism</p></p></p>
<p><p><p>Incorporated into DNA, which inhibits synthesis and function
Inhibits ribonucleotide reductase (RNR), which reduces precursors for DNA
</p></p></p>
<p><p><p>6-MP and 6-TG
| Mechanism</p></p></p>
<p><p><p>Incorporated into DNA, which inhibits synthesis and function
Reduce precursors for RNA and DNA by inhibiting purine synthesis
</p></p></p>
<p><p>Cladribine
| Mechanism</p></p>
<p><p>Incorporated into DNA
Causes strand breaks
Potent inhibitor of ribonucleotide reductase (RNR), which reduces DNA precursors
</p></p>
<p><p><p>Cladribine
| Mechanism</p></p></p>
<p><p><p>Incorporated into DNA
Causes strand breaks
Potent inhibitor of ribonucleotide reductase (RNR), which reduces DNA precursors
</p></p></p>
<p></p>
<p>General mechanisms of alkylating agents</p>
<p></p>
<ul>
<li>Among the oldest and most useful class</li>
<li>Reactions between alkyl groups on drug with nucleophilic groups on proteins and nucleic acids</li>
<li>Most common binding site is seven-nitrogen group of guanine</li>
<li>Cause DNA crosslinking and strand breakage</li>
</ul>

<p><p>Alkylating agents</p></p>
<p><p>Cytotoxic cell cycle nonspecific agents (they are toxic in all stages of cell cycle)
</p></p>
<p><p>Common alkylating agents</p>
| </p>
<p><ul>
<li>Nitrogen mustards
<ul>
<li>Related to the ‘mustard gas’ used during the First World War</li>
<li>Examples include: <strong>Mechlorethamine, cyclophosphamide</strong>, chlorambucil, and ifosfamide</li>
</ul>
</li>
<li><span></span>Nitrosoureas
<ul>
<li><strong>Carmustine (BCNU)</strong> and lomustine</li>
</ul>
</li>
</ul>
<p></p>
<p></p>
</p>
<p></p>
<p>Cyclophosphamide (4)</p>
<p></p>
<ul>
<li>Cytotoxic Agent</li>
<li>Alkylating agent</li>
<li>Nitrogen Mustard</li>
<li>most commonly used alkylating agent in both solid tumors and hematological malignancies.</li>
<li>Hemorrhagic cystitis caused by acrolein (5-10% of patients)</li>
</ul>

<p></p>
<p>mesna</p>
<p></p>
<ul>
<li>Co-administration of mesna (with cyclophosphamide), a sulfhydryl compound, <strong>inactivates acrolein</strong> (cytotoxic to bladder cells).</li>
<li>Reduces risk of hemorrhagic cystitis</li>
</ul>
<p>Resistance to alkylating agents</p>
<p>Inactivation by glutathione and other nucleophiles (increased glutathione production)
Reduced uptake
Accelerated DNA repair
Increased expression of O6-methylguanine-DNA methyltransferase (MGMT)
MGMT prevents permanent DNA damage by removing alkyl groups from guanine before cross-links form
</p>

<p><p>Resistance to alkylating agents</p>
| </p>
<p><ul>
<li>Inactivation by glutathione and other nucleophiles (increased glutathione production)</li>
<li>Reduced uptake</li>
<li>Accelerated DNA repair</li>
<li>Increased expression of O6-methylguanine-DNA methyltransferase (MGMT)
<ul>
<li>MGMT prevents permanent DNA damage by removing alkyl groups from guanine before cross-links form</li>
</ul>
</li>
</ul>
</p>
<p>Non-classical alkylating agents </p>
<p>(Platinum compounds)
Considered non-classical alkylating agents because while they lead to DNA cross-linkages, they have no alkyl group like the classical alkylating agents.
Targets nucleophilic center (primarily at guanine-N7
Actively transported into cells via a Cu2+ transporter
</p>
<p>Cisplatin</p>
<p>1st generation platinum agent cytotixic agent non-classical alkylating agent platinum analog </p>
<p>Carboplatin </p>
<p>2nd generation platinum agent cytotixic agent non-classical alkylating agent platinum analog </p>
<p>Oxaliplatin</p>
<p>3rd generation platinum agent
cytotixic agent
non-classical alkylating agent
platinum analog</p>
<p>Non-classical alkylating agents
| Commonly used to </p>
<p>ovarian, testicular, head and neck, bladder, esophagus, lung, and colon cancers
</p>
<p>Cisplatin
| adverse effects</p>
<p>Anaphylactic-like reactions (hypersensitivity reactions)
Peripheral motor and sensory neuropathy
Nephrotoxicity
Can be significantly reduced by increasing hydration by co-administering intravenous saline
</p>
<p>Cisplatin
| adverse effects</p>
<p>Anaphylactic-like reactions (hypersensitivity reactions)
Peripheral motor and sensory neuropathy
Nephrotoxicity
Can be significantly reduced by increasing hydration by co-administering intravenous saline
</p>
<p>Vinblastine</p>
<ul>
<li>Cytotoxic agent</li>
<li>Plant dericatives and similar compounds</li>
<li>vinca alkaloid (from periwinkle plant)</li>
<li>Antimicrotubule agents</li>
</ul>
<p>Vincristine</p>
<ul>
<li>Cytotoxic agent</li>
<li>Plant dericatives and similar compounds</li>
<li>vinca alkaloid (from periwinkle plant)</li>
<li>Antimicrotubule agents</li>
</ul>

<p>Adverse effects of vincristine
| </p>
<p>Mostly neurological (numbness and tingling in extremities, motor weakness)
</p>
<p>Paclitaxel (taxol)</p>
<ul> <li>Cytotoxic agent</li> <li>Plant derivatives and similar compounds</li> <li>Taxanes</li> <li>Antimicrotubule agents</li> </ul>

<p>Paclitaxel (taxol) mechanism</p>
<p>Paclitaxel kills tumor cells by arresting them in mitosis by preventing the depolymerization of microtubules.
</p>
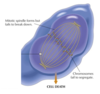
<p>Paclitaxel (taxol) adverse effect </p>
<p>peripheral neuropathy
| </p>
<p>filgrastim</p>
<ul>
<li>(granulocyte-colony stimulating factor) used often with Paclitaxel to reduce myelosuppression</li>
<li>Hypersensitivity allergic reactions occur in about 5 % of the patients receiving paclitaxel.
<ul>
<li>This is largely prevented when patients are pretreated with dexamethasone and anti-histamines.</li>
</ul>
</li>
</ul>
<p>irinotecan</p>
<ul>
<li>cytotoxic agent</li>
<li>plant derivative and similar compound</li>
<li>camptothecins analog</li>
<li>induce cytotoxicity by inhibiting topoisomerase I (prevents repair of cuts leading to DNA damage).</li>
</ul>
Etoposide
cytotoxic agent
plant derivative and similar compound
other
is a class II topoisomerase inhibitor
<p>Etoposide</p>
<ul>
<li>cytotoxic agent</li>
<li>plant derivative and similar compound</li>
<li>other</li>
<li>is a class II topoisomerase inhibitor</li>
</ul>
Doxorubicin
cytotoxic antibiotics
anthracyclines (anthracycline antibiotic)
Doxorubicin mech
It intercalates with DNA, leading to the inhibition DNA polymerase.
Inhibits topoisomerase II
Cause DNA double-strand breaks, which can lead to cell death
Doxorubicin binds to iron and generates free radicals, which lead to DNA and protein damage.
Free radical formation causes adverse effects, but not thought to be the major mechanism of tumor cell killing,
Doxorubicin Adverse effect
Irreversible cardiomyopathy
Bleomycin
Cytotoxic Anitibiotics
Bleomycin mech
Small peptide that binds to DNA and causes single and double strand breaks
Cell cycle specific drug (causes cells to arrest in G2 phase)
Importantly, bleomycin is minimally myelosuppressive and immunosuppressive (used in combination therapy with other cytotoxic drugs)
Bleomycin used in
curative combination chemotherapy regimens for testicular cancer and Hodgkin’s disease
Bleomycin Adverse effect
Dose-limiting adverse side effect is pulmonary toxicity
Effects are cumulative and irreversible
The majority of cytotoxic agents induce
myelosuppression
Prednisone
hormone
glucocorticoid
Inhibit lymphocyte proliferation
Used to treat leukemias and lymphomas
Useful in reducing intracranial pressure associated with brain tumors
Useful to reduce adverse effects of chemotherapeutics such as nausea and vomiting
dexamethasone
hormone
glucocorticoid
Inhibit lymphocyte proliferation
Used to treat leukemias and lymphomas
Useful in reducing intracranial pressure associated with brain tumors
Useful to reduce adverse effects of chemotherapeutics such as nausea and vomiting
Tamoxifen
Partial estrogen receptor antagonist
Selective estrogen receptor modulator (SERM)
Tamoxifen is a non-steroidal that competitively binds to estrogen receptor and reduces the growth of estrogen dependent breast cancers
Flutamide
Androgen receptor antagonists that are useful for the treatment of prostate cancer
These drugs prevent dihydrotestosterone from binding to androgen receptors
Anastrozole
Aromatase inhibitor
Post-menopausal women synthesize estrogen from peripheral tissue where aromatase converts testosterone into estrogen.
Anastrozole inhibits aromatase activity, which can lower estrogen levels.
Anastrozole
Used to treat
estrogen-sensitive (estrogen receptor-positive) breast tumors in post-menopausal women
Trastuzumab (Herceptin)
monoclonal antibodies
HER-2 Inhibitor
Used to treat breast cancer with HER-2 amplified
First monoclonal antibody approved for the treatment of solid tumors
Trastuzumab (Herceptin) Toxicities
Most serious is cardiotoxicity
Trastuzumab (Herceptin) Toxicities
Most serious is cardiotoxicity
Cetuximab
Targeted agent
Monoclonal antibody that binds to EGFR and blocks signaling
Used to treat EGFR-expressing colorectal tumors in combination with other drugs
Also used in combination with radiation therapy in head and neck cancers
Activating mutations in RAS in colorectal tumors cause cells to be resistant
Routine tests of RAS mutational status are performed
Bevacizumab
Bevacizumab is an monoclonal antibody directed against vascular endothelial growth factor (VEGF).
Bevacizumab Mech
binds to VEGF, which prevents VEGF from binding to VEGFR (prevents angiogenesis).
Bevacizumab Uses
Colorectal cancer in combination with capecitabine and oxaliplatin
It is also used in combinations with other drugs to treat metastatic breast and colorectal cancer.
Lapatinib
Small Molecule tyrosine kinase inhibitor
Lapatinib Mech
Small molecule that inhibits both EGFR and HER-2 kinase activity
Lapatinib Used in combination
with capecitabine to treat HER2-amplified, trastuzumab-refractory breast cancer
Erlotinib
Small molecule turosine kinase inhibitor
Oral small-molecule EGFR inhibitor
ATP competitive inhibitor
Erlotinib First-line treatment of
metastatic nonsmall cell lung carcinoma (NSCLC) in patients with EGFR exon 19 deletions or exon 21 (L858R) substitution mutations
Need to determine mutation status with and FDA approved test
Resistance often occurs due to acquired secondary mutation in EGFR or by amplification of the MET oncogene.
Imatinib
Small molecule tyrosine kinase inhibitor
Imatinib (Gleevec) is a small molecular inhibitor of BCR-ABL
Imatinib (and related compounds) induces remission (both clinical and molecular) in greater than 90 % of patients in the chronic phase of the disease.
Chronic myelogenous leukemia (CML) is caused by
the Philadelphia chromosome translocation.
Fusion protein between BCR and the ABL tyrosine kinase
Leads to constitutive activation of ABL
Imatinib Resistance mechanisms
Point mutations in BCR-ABL cause a reduced affinity for imatinib
Fortunately, the analogs of imatinib (nilotinib and dasatinib) can still inhibit BCR-ABL with many of these mutations
Asparaginase
Miscellaneous
Enzyme used to treat childhood acute lymphoblastic leukemia (ALL)
Hydrolyzes plasma L-asparagine into L-aspartate
While normal cells can synthesize sufficient L-asparagine, tumor cells cannot.
Thus, asparaginase can starve tumor cells of L-asparagine.
Asparaginase adverse effect
allergic hypersensitivity reaction (fever, chills, rash, hives)
Can become severe causing respiratory failure and hypotension
Bortezomib
(proteasome inhibitor)
Bortezomib Mech
By inhibiting the proteasome, it elevates levels of p53
Can also reduce levels of a protein that normally inhibits apoptosis called NF-kappaB
Bortezomib Approved for treatment of
patients with relapsed or refractory multiple myeloma
Bortezomib Adverse effects:
Peripheral neuropathy is most the most chronic toxicity
Temsirolimus
Used to treat renal cell carcinoma
Inhibition of mTOR complex 1 (mTORC1) reduces protein translation, promotes cell cycle inhibition, and promotes apoptosis.
Temsirolimus Resistance mechanism
mTOR forms a complex called mTOR complex 2 (mTORC2)
mTORC2 is not inhibited by temsirolimus
Adverse effect: Nephrotoxic
cisplatin, methotrexate
Adverse effect: Neurotoxic (peripheral neuropathies, cerebellar syndrome, and others
vincristine, cytarabine (ara C), cisplatin, bortezomib, paclitaxel
Adverse effect: Cardiac toxicity
doxorubicin,
trastuzumab
Adverse effect: Pulmonary
toxicities
methotrexate, bleomycin,
alkylating
agents
Adverse effect: Bladder toxicity (hemorrhagic
cystitis)
cyclophosphamide
Adverse effect: Hypersensitivity reactions
asparaginase,
paclitaxel
Mechlorethamine
Cytotoxic agent
alkylating agent
nitrogen mustards
Related to the ‘mustard gas’ used during the First World War
“Cytotoxic cell cycle nonspecific agents (they are toxic in all stages of cell cycle)
“
Oxaliplatin
Considered non-classical alkylating agents because while they lead to DNA cross-linkages, they have no alkyl group like the classical alkylating agents.
Targets nucleophilic center (primarily at guanine-N7
(3rd generation)
Actively transported into cells via a Cu2+ transporter
Commonly used to ovarian, testicular, head and neck, bladder, esophagus, lung, and colon cancers
dexrazoxane
Iron chelator
used with Doxorubicin to reduce cardiotoxicity
Aspirin (mechanism of action)
Aspirin irreversibly blocks cyclooxygenase -1 (COX-1) in platelets.
Reduces thromboxane A2 production (which leads to reduced platelets activation and aggregation).
Other agents that inhibit COX-1 reversibly do not have antiplatelet effects (e.g., ibuprofen).
Dipyridamole
Elevated cAMP levels reduce intracellular Ca2+ levels (reduce activation of platelets).
Dipyridamole is a vasodilator that can be used in combination with warfarin to inhibit embolization from mechanical heart valves.
By itself, dipyridamole has little anti-thrombotic effects.
Clopidogrel
irreversible P2Y12 inhibitors that are prodrugs that have to be activated by metabolism in the liver
has to be activated by CYP2C19
prasugrel
irreversible P2Y12 inhibitors that are prodrugs that have to be activated by metabolism in the liver
Ticagrelor
P2Y12 inhibitors
do not need to be metabolized to become activated.
Is reversible
More rapid coagulation recovery upon discontinuation
cangrelor
P2Y12 inhibitors
do not need to be metabolized to become activated.
Is reversible
More rapid coagulation recovery upon discontinuation
Abciximab
typically used as adjunct therapy in patients undergoing PCI to prevent ischemic complications.
Sometimes combined with heparin and aspirin as adjunct to PCI
eptifibatide
typically used as adjunct therapy in patients undergoing PCI to prevent ischemic complications.
Sometimes combined with heparin and aspirin as adjunct to PCI
Tirofiban and eptifibatide
used in patients with unstable angina.
Abciximab Mech
Fragment of antigen-binding (Fab) segment of a monoclonal antibody directed against GPIIb/IIIa
The binding of abciximab to GPIIb/IIa prevents platelet aggregation by preventing the fibrinogen cross-bridges from forming between platelets.
Abciximab also binds to receptors related to GPIIb/IIIa on leukocytes, which might account for the added antiinflammatory and antiproliferative effects of abciximab
Eptifibatide Mech
a peptide that binds to and inhibits GPIIb/IIIa.
Tirofiban Mech
a nonpeptidic small molecule that binds to and inhibits GPIIb/IIIa.
Both _______ and _______ have a lower risk of producing thrombocytopenia than abciximab.
eptifibatide and tirofiban
Vorapaxar
Protease activated receptor (PAR) antagonist
Recently approved by FDA for the prevention of thrombotic cardiovascular events in patients with a history of myocardial infarction (MI) or with peripheral arterial disease
Indirect inhibitors of thrombin and/or Factor Xa (anticoagulant effect exerted through binding to antithrombin)
Heparin (parenteral)
Enoxaparin (low molecular weight heparin) (parenteral) *
Fondaparinux (parenteral) *
Direct thrombin inhibitors
Lepirudin (parenteral)
Bivalirudin (parenteral)
Argatroban (parenteral)
Dabigatran (oral)**
Direct factor Xa inhibitors
Rivaroxaban (oral) **
Apixaban (oral) **
Vitamin K antagonists (VKAs)
Warfarin (oral)
Heparin sulfate
is a proteoglycan found on the surface of vascular endothelial cells.
Binds to antithrombin to increase inhibition of factor Xa and thrombin
Heparin (mechanism of action)
A specific pentasaccharide sequence in heparin binds to antithrombin (AT)
Changes conformation of AT causing it to have a higher affinity for factor Xa
This accelerates the rate of factor Xa inhibition without affecting thrombin inhibition.
Heparin can also increase AT-induced inhibition of thrombin.
It does this by acting as a molecular bridge that brings thrombin into close contact with AT.
Only longer heparin molecules can facilitate this .
Heparin is a family
sulfated-polysaccharides of varying molecular weights found in mast cells and thought to be required for histamine storage.
Not normally found in the plasma
Heparin can be isolated from
tissues rich in mast cells (usually animal intestines or lungs)
heparin Administered
parenterally to inhibit coagulation
Heparin (pharmacokinetics)
Heparin has to be administered parenterally.
To achieve anticoagulant effect rapidly, heparin is usually administered intravenously.
After heparin enters circulation
Binds not only to antithrombin, but other plasma proteins, and endothelium of vessel walls
Interaction with plasma proteins other than antithrombin reduces anticoagulant activity
Acute-phase proteins (plasma proteins that change in response to inflammation), which can be elevated in ill patients
Platelet factor 4 (PF4) (secreted by platelets)
Levels of heparin binding proteins differ from person to person cause fixed doses to be unpredictable.


