✅☢ CRX Flashcards
- Firsts, 3. Quality, 4. Airway, 5. Bone, 6. CadioMediastinum 7. Diaohragm, 8. Effusions (Pleural, pericardial), 9. Fields (lung), 10. Pneumonia, 13. Groung glass, SPN, TB, PH, PE, Pneumo, IPF, emohysema, Mediastinal mass, pneumopericardium, dia hernia, hilar adenopathy, LC, abscess, tubes lines drains, pleural effusion, pericardial effusion, pulmonary edema, cadiogenic edema, CHF, cephalization, kerley b, interstitial edema, air bronchogram, alveolar edema, ARDS, atelectasis, 64., 67. aspiration syndromes
Index
Firsts
Turn off stray lights, optimize room lighting, view images in order
Patient data:
Name
Date
PA/AP
Uprigt/supine
Quality
Rotated? Symmetrical clavicles / clavicular heads
Penetration? Thoracic spine seen through heart
All areas included? (costophrenic angles)
Inflation / Inspiratory effort (3 cm diaphragmatic curvature; 8 (9) -10 posterior ribs in nl)
Airway
Midline / Deviation of the trachea (related to a mediastinal hematoma)
Masses in the airway
Airway compression
Bone
Lesions or fractures
Clavicles
Scoliosis?
Soft tissue calcification
RUG (gallstones, free air)
CardioMediastinum
Borders (R, aortic knob, pulmonary a, l. atrium, l ventricle)
Lines (R paratracheal, Azygous, SVC, Aorta, Azygoesophageal line, descending aortic line)
Cardiomegaly (Cardiothoracic ratio)
RA, LV, LA
Mediastinal contour: width? mass?
Widened mediastinum:
Loss of the normal clear aortic arch contour “knob”
Loss of the appearance of a normal descending thoracic aorta (no ‘lateral aortic silhouette’ is seen).
Deviation of nasogastric tubes to the right (Indicating a mediastinal hematoma pushing the esophagus to the right side).
Left apical pleural ‘capping’
There is is a rind of fluid above the left lung apex where blood has tracked posteriorly over the left apex.
Signs of a supine pleural effusion
Diaphragm
Sharp border
Costophrenic angles sharp bilaterally
Air under diaphragm
Effisions (Pleura, Pericardial)
Pleural, pericardial
Lucencies (pneumothorax)
Thickeing, nodularity, calcification, or effusions
Fields (Lung)
Lung zones symmetrical?
Parenchyma (focal or diffuse abnormalitis)
Interstitial and vascular markings (size, prominence)
Lucency (pneumothorax), cavity, or abnormal shadowing (companion shadow of the second rib)
Hila (l higer than right; branching pattern)
Infiltrates
Alveolar process (fluffy)[filled alveoli]
Reticular process (lacy)[cell]
Pneumonia
Pneumonia is a space occupying lesion without volume loss. Pneumonia is caused by bacteria, viruses, mycoplasmae and fungi.
Ddx: Airspace filling not distinguishable radiographically: fluid (inflammatory), cells (cancer), protein (alveolar proteinosis) and blood (pulmonary hemorrhage).
The x-ray findings of pneumonia are airspace opacity, lobar consolidation, or interstitial opacities. There is usually considerable overlap.
Lobar - classically Pneumococcal pneumonia, entire lobe consolidated and air bronchograms common
An “Air bronchogram” is a tubular outline of an airway made visible by filling of the surrounding alveoli by fluid or inflammatory exudates. Six causes of air bronchograms are: Lung consolidation (PNA), pulmonary edema, nonobstructive pulmonary atelectasis, severe interstitial disease, neoplasm, and normal expiration.
Lobular - often Staphlococcus, multifocal, patchy, sometimes without air bronchograms
Interstitial - Viral or Mycoplasma; latter starts perihilar and can become confluent and/or patchy as disease progresses, no air bronchograms
“Ground Glass” is a radiology descriptive term (used in both chest radiographs and CT imaging) to indicate that blood vessels are not obscured as would be the case in alveolar lung opacities. Both atypical bacterial and viral organisms may produce pneumonias that differ radiographically from more common bacteria such as pneumococcus. They may produce a ground glass appearance and increased interstitial markings. The CXR appearance of Pneumocystis pneumonia is typically bilateral, diffuse interstitial (“reticular”) or ground glass opacities.
Aspiration pneumonia - follows gravitational flow of aspirated contents; impaired consciousness, post anesthesia, common in alcoholics, debilitated, demented pts; anaerobic (Bacteroides and Fusobacterium)
Diffuse pulmonary infections - community acquired (Mycoplasma, resolves spontaneoulsy) nosocomial (Pseudomonas, debilitated, mechanical vent pts, high mortality rate, patchy opacities, cavitation, ill-defined nodular) immunocompromised host(bacterial, fungal, PCP)
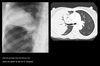
Pneumonia in the ICU
Nosocomial pneumonias by definition occur 3 days after admission. They differ from community-acquired pneumonias in both etiology and prognosis. Patients in the ICU are often relatively immunocompromised secondary to their primary disease and are subject to iatrogenic factors which increase their sucseptabilty to pneumonia-causing pathogens. These include the following: endotracheal tubes, which defeat many patient defense mechanisms; medications used to reduce gastric acid, which may promote bacterial growth in the stomach; and the use of antibiotics, which may selectively encourage the growth of some pathogenic bacteria. Nosocomial pneumonia presents a great concern for the intensivist and is the leading cause of infectious death in hospitals. Unlike community-acquired pneumonias, which usually are caused by gram-positive species, nosocomial pneumonias are often polymicrobial and caused by gram-negative enteric pathogens. The offending organisms often include Pseudomonas species, E-coli, Klebsiella species, and Proteus species. Traditional clinical indicators of pneumonia, including fever, elevated white blood cell count, and positive sputum cultures are often masked by severe underlying disease. The chest film must be correlated with clinical data in order to make the diagnosis of pneumonia in the ICU patient.
In a supine patient who has aspirated, the common locations of pneumonia are the psoterior segment of the upper lobe and superior segment of the lower lobe
Radiographic Appearance of Pneumonia
The radiographic appearance of pneumonia may be difficult to differentiate from atelectasis or early ARDS. Classically, pneumonia first appears as patchy opacifications or ill-defined nodules. It is often multifocal and bilateral, occurring most often in the gravity dependent areas of the lung. This feature makes it difficult to distinguish from atelectasis or apulmonary edem. E-coli and pseudomonas species can rapidly involve the entire lung. Their symmetric pattern often simulates pulmonary edema. The presence of patchy air space opacities, air bronchograms, ill-defined segmental consolidation or associated pleural effusion support the diagnosis of pneumonia. Occassionally, in gram-negative pneumonias small luciencies may be found within consolidated lung which may represent unaffected acini or areas of air trapping. This is particularly likely to occur in patients with underlying COPD. However, these must be distinguished from lucencies created by cavitation and abscess formation.
Complications of nosocomial pneumonias can have severe consequences and require immediate attention. Unlike community acquired pneumonia, pleural effusions caused by gram-negative organisms are more likely to represent empyema and therefore require drainage. Other complications of nosocomial pneumonias include lung abscess formation and bronchopleural fistulas.

Solitary Pulmonary Nodule
A differential of possible etiologies is as follows:
Granuloma – usually caused by fungal infections like histoplasmosis or tuberculosis
Lung Carcinoma
Solitary metastasis – usually from colon, breast, kidney, ovary, or testis
Round pneumonia
Abscess
Round atelectasis
Hamartoma – popcorn calcification is sometimes seen
Sequestration
Arteriovenous malformation
Other things can cause an apparent nodule but are actually outside the lung including:
Fluid in an interlobar fissure
Pleural plaques – small, often calcified, plate-like surfaces on the pleura often caused by asbestos fibers that invade the pleura from the lungs
Skin lesions – nipple shadow, mole, lipoma, etc.
Low Risk Patient
≤ 4mmNo follow-up needed
4-6mm12 mo; if no change - stop
6-8mm6-12 mo; no change - follow-up at 18-24 mo
> 8mmCT follow-up at 3, 9, 24mo or PET/CT, or biopsy
High Risk Patient (eg. smoking history or history of malignancy)
≤ 4mm 12 mo; if no change - stop
4-6mm 6-12mo; no change - follow-up at 18-24 mo
6-8mm 3-6mo; no change - follow-up at 18-24 mo
> 8mm CT follow-up at 3, 9, 24mo or PET/CT, or biopsy
Post-primary TB:
Focal patchy airspace disease “cotton wool” shadows, cavitation, fibrosis, nodal calcification, and flecks of caseous material. These occur most commonly in the posterior segments of the upper lobes, and superior segments of the lower lobes.
Pulmonary hemorrhage:
Blood fills the bronchi and eventually the alveoli.
Has an appearance like that of other airspace filling processes (pneumonia, edema) which have opacity often with air bronchograms.
Caused by trauma, Goodpastrue’s syndrome, bleeding disorders, high altitude, and mitral stenosis.
Notable in that it may clear more quickly than other alveolar densities such as pneumonia.
Pulmonary Embolism
Even though pulmonary embolism is the third most common cause of sudden death, it continues to be underdiagnosed in the intensive care setting. The clinical manifestations of pulmonary embolism are varied. They range from completely silent embolization to sudden death. Symptoms of dyspnea, tachypnea, hemoptysis, hypoxemia, and pleuritic chest pain have been attributed to pulmonary embolism but are neither sensitive nor specific. Indeed, the most valuable indicators of pulmonary embolism are a history of risk factors and or a previous embolic event. Many different medical and surgical conditions are associated with increased risk of pulmonary embolization, including immobilization, trauma, surgery, shock, obesity, pregnancy, polycythemia vera, and antithrombin-III deficiency. The pathophysiology of pulmonary embolism consists of both hemodynamic and respiratory embarrassment. Approximately 90% of pulmonary embolisms are the result of venous thrombosis in the lower extremities. Hemodynamic consequences occur when more than half the cross sectional area of the pulmonary vascular bed is occluded. This situation leads to pulmonary hypertension and in the acute setting right heart failure. Increased alveolar dead space (a result of ventilated but underperfused lung) leads to hypoxemia and respiratory failure. Pulmonary infarction is a rare consequence of pulmonary embolism in patients without concommitent compromise of the bronchial circulation. Generally, infarctions are hemorrhagic and located in the lower lobes.
Radiographic Findings in Pulmonary Embolism (PE)
Due to its relative lack of sensitivity, the chest x-ray in patients with suspected pulmonary embolism is usually relegated to the role of ruling out other disorders which may have a similar clinical presentation. The chest x-ray is also very useful when interpreting ventilation-perfusion scans. Though the majority of patients with pulmonary embolism in retrospect do have abnormalities on the chest x-ray, findings are usually too non-specific to be of diagnostic value. Without infarction there are few chest film signs of pulmonary emboli. These include discoid atelectasis, elevation of the hemidiaphragm, enlargement of the main pulmonary artery into what has been described as the shape of a “sausage” or a “knuckle” (Palla’s sign), and pulmonary oligemia beyond the point of occlusion (Westermark’s sign). Occasionally, pulmonary embolisms will cause infarction causing a unique constellation of radiographic signs. Multifocal consolidation of the affected lung may occur in 12 to 24 hours following the embolic event. A consolidation which begins at the pleural surface and is rounded centrally is called a Hamptom’s Hump. These types of consolidation differ from pneumonia in that they lack air bronchograms. Up to 50% of patients with pulmonary embolism will also have ipsilateral or bilateral pulmonary effusions, although these are certainly nonspecific findings. Nevertheless, it is unusual for pulmonary infarctions to be diagnosed by chest radiography although infarctions are known to occur much more frequently. Presumably infarcts are confused with or indistinguishable from atelectasis or pneumonia. Despite the low sensitivity of these signs, the chest radiograph remains an important first step in the diagnosis of pulmonary embolism, primarily to exclude other causes of hypoxemia and to aid in the interpretation of the ventilation/perfusion scan.
May see: Westermark’s sign (oligemia in area of involvement), increased size of a hilum (caused by thrombus impaction), atelectasis with elevation of hemidiaphragm and linear or disk shaped densities, pleural effusion, consolidation, and Hampton’s hump (rounded opacity). In the case of pulmonary infarctions, the main radiographic feature is multifocal consolidation at the pleural base in the lower lungs.
Several other important modalities are used when investigating possible PE. These modalities are venous ultrasound, V/Q scan, pulmonary arteriogram, and CT angiogram (CTA). Remember, if the CXR of a patient with hypoxia is normal you should consider PE.
The workup of suspected PE can be divided into two populations. In the inpatient setting a CTPA will likely be more definitive than a V/Q scan, as it may disclose other causes of hypoxia not shown on CXR. If the patient has leg swelling, a venous ultrasound of the leg veins should be done to exclude DVT. In the outpatient setting a V/Q scan should be the first test and will less likely be indeterminate than in the inpatient setting. There is also a lower radiation dose for V/Q scans than for CTPA. If these studies are inconclusive a pulmonary arteriogram is the definitive, but more invasive test.

Pneumothorax
Defined as air inside the thoracic cavity but outside the lung (pleural space).
Some causes of spontaneous PTX are; idiopathic, asthma, COPD, pulmonary infection, neoplasm, Marfan’s syndrome, and smoking cocaine. However, most pneumothoraces are iatrogenic and caused by a physician during surgery or central line placement. Trauma, such as a motor vehicle accident is another important cause. Pneumothorax is a common complication of invasive procedures such as central line placement, especially in the mechanically ventilated patient. Barotrauma also can lead to pneumothorax, complicating the intubated patient’s medical course. The air may also arrive at the intrapleural space by rupture of alveoli (blebs), extension of a pneumomediastinum, or communication with extrathoracic air following trauma or surgery.
A tension PTX is a type of PTX in which air enters the pleural cavity and is trapped during expiration usually by some type of ball valve-like mechanism. This leads to a buildup of air increasing intrathoracic pressure. Eventually the pressure buildup is large enough to collapse the lung and shift the mediastinum away from the tension PTX.
On CXR, a PTX appears as air without lung markings in the least dependant part of the chest. Generally, the air is found peripheral to the white line of the pleura. In an upright film this is most likely seen in the apices. A PTX is best demonstrated by an expiration film. It can be difficult to see when the patient is in a supine position. In this position, air rises to the medial aspect of the lung and may be seen as a lucency along the mediastinum. It may also collect in the inferior sulci causing a deep sulcus sign.
A hydropneumothorax is both air and fluid in the pleural space. It is characterized by an air-fluid level on an upright or decubitus film in a patient with a pneumothorax.
Some causes of a hydropneumothorax are trauma, thoracentesis, surgery, ruptured esophagus, and empyema.
Px: Hypotension

Apicolateral pneumothorax
Appears as a thin, white pleural line with no lung markings beyond. The presence of lung markings beyond this line, though, does not exclude pneumothorax. This is especially true in the patient with parenchymal disease which may alter the compliance of affected lobes, making their collapse more difficult to detect radiographically. Parenchymal disease may also make visualization of the pleural line more difficult or impossible.

Pneumothorax in the Supine Patient
In the supine patient, intrapleural air rises anteriorly and medially, often making the diagnosis of pneumothorax difficult. The anteromedial and subpulmonary locations are the initial areas of air collection in the supine patient. An apical pneumothorax in a supine patient is a sign that a large volume of air is present. Subpulmonic pneumothorax occurs when air accumulates between the base of the lung and the diaphragm. Anterolateral air may increase the radiolucency at the costophrenic sulcus. This is called the deep sulcus sign. Other signs of subpulmonic pneumothorax include a hyperlucent upper quadrant with visualization of the superior surface of the diaphragm and visualization of the inferior vena cava.

Subpulmonary Pneumothorax
Occasionally, a posterior subpulmonary pneumothorax will result in visualization of the more superior anterior diaphragmatic surface and the inferior posterior diaphragmatic surface, resulting in the double-diaphragm sign.

Anteromedial Pneumothorax
Anteromedial pneumothoraces are differentiated into those which are superior or inferior to the pulmonary hilum. A superior anteromedial pneumothorax may result in visualization of the superior vena cava or azygos vein on the right. An inferior anteromedial pneumothorax may be evidenced by delineation of the heart border and a lucent cardiophrenic sulcus. This is the key sign of a pneumothorax as this is the highest point in the supine patient, where the air will accumulate first.

Tension Pneumothorax
Mediastinal shift is usually seen in a tension pneumothorax, but the use of PEEP may prevent this from occurring. The most reliable sign of tension pneumothorax is depression of a hemidiaphragm. Other signs of tension pneumothorax include shifting of the heart border, the superior vena cava, and the inferior vena cava. The shifting of these structures can lead to decreased venous return.
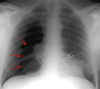
Pneumomediastinum
In the intubated patient the most likely source of air in the mediastinum is pulmonary interstitial air dissecting centripetally. Air in the mediastinum may also originate from tracheobronchial injury or air dissecting through fascial planes from the retroperitoneum. A sudden increase in thoracic pressures (e.g. blunt trauma) may also cause alveolar rupture and consequently pneumomediastinum.
Findings include; streaky lucencies over the mediastinum that extend into the neck, and elevation of the parietal pleura along the mediastinal borders.
Pneumomediastinum often dissects up into the neck. This helps to distinguish it from pneumopericardium that, unlike pneumomediastinum, can extend inferior to the heart.
Causes of pneumomediastinum include; asthma, surgery (post-op complication), traumatic tracheobronchial rupture, abrupt changes in intrathoracic pressure (vomiting, coughing, exercise, parturition), ruptured esophagus, barotrauma, and smoking crack cocaine.
Pneumomediastinum should be distinguished from pneumopericardium and pneumothorax. In pneumopericardium, air can be present underneath the heart, but does not enter the neck.
Continuois diaphram sign
Pneumomediastinum generally will not develop clinical manisfestations. However, a retrosternal crunch is sometimes auscultated (Hamman’s crunch).
Pneumomediastinum rarely causes tension pericardium due to the compressibility of air and the fact that rarely is the pneumomediastinum non-communicating tension due to air is rare. Pneumomediastinum may cause pneumothorax (the reverse is not true) or pneumoperitoneum.

Interstitial pulmonary fibrosis
The six most common causes of diffuse interstitial pulmonary fibrosis are idiopathic (IPF, >50% of cases), collagen vascular disease, cytotoxic agents and nitrofurantoin, pneumoconioses, radiation, and sarcoidosis. Clinically the patient with IPF will present with progressive exertional dyspnea and a nonproductive cough. Radiographically, IPF is associated with hazy “ground glass” opacification early and volume loss with linear opacities bilaterally, and honeycomb lung in the late stages. IPF carries a poor prognosis with death due to pulmonary failure usually occurring within 3-6 years of the diagnosis unless lung transplant is performed.
Emphysema
Loss of elastic recoil of the lung with destruction of pulmonary capillary bed and
Commonly seen on CXR as diffuse hyperinflation with flattening of diaphragms, increased retrosternal space, bullae (lucent, air-containing spaces that have no vessels that are not perfused) and enlargement of PA/RV (secondary to chronic hypoxia) an entity also known as cor pulmonale. Hyperinflation and bullae are the best radiographic predictors of emphysema. However, the radiographic findings correlate poorly with the patientâs pulmonary function tests. CT and HRCT (high resolution CT) has emerged as a technique to evaluate different types, panlobular, intralobular, paraseptal and for guidance prior to volume reduction surgery.
Occasionally the trachea is very narrow in the mediolateral plane in emphysema. “Saber sheath” tracheal deformity is when the coronal diameter is less than 2/3 that of the sagittal.
In smokers with known emphysema the upper lung zones are commonly more involved than the lower lobes. This situation is reversed in patients with alpha-1 anti-trypsin deficiency, where the lower lobes are affected.
Chronic bronchitis commonly occurs in patients with emphysema and is associated with bronchial wall thickening.
alveolar septa.
“loculated”
Anterior Mediastinal Mass
Consist of the 4 “T’s” (Terrible lymphadenopathy, Thymic tumors, Teratoma, Thyroid mass) and aortic aneurysm, pericardial cyst, epicardial fat pad. Usually CT or fine needle aspiration is needed to make the definitive diagnosis of an anterior mediastinal mass.
Middle mediastinal mass
Common cause is lymphadenopathy due to metastases or primary tumor. Other causes include hiatial hernia, aortic aneurysm, thyroid mass, duplication cyst, and bronchogenic cyst.
Posterior Mediastinal Mass
Ddx includes neoplasm, lymphadenopathy, aortic aneurysm, adjacent pleural or lung mass, neurenteric cyst or lateral meningocele, and extramedullary hematopoiesis.
Pneumopericardium
Pneumopericardium, an uncommon occurence, is most often found in the post-operative cardiac patient. Radiographically, pneumopericardium appears as a lucent area around the heart extending up to the main pulmonary arteries.
A lucent stripe along the inferior border of the cardiac silhouette which crosses the midline is also diagnostic for pneumopericardium “continuous diaphragm sign”
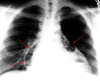
Diaphragmatic hernia
There are 3 types of diaphragmatic hernia that may be seen in CXR. By far the most common is a hiatal hernia - the stomach slips through the esophageal hiatus into the chest. A Bochdalek hernia is through a weakness in the diaphragm, and usually occurs on the left side posteriorly (Bochdalek - back and to the left). Morgagni hernias typically occur medially. Weakness of the diaphragm can occur without frank herniation of abdominal contents. This is termed an eventration, and it usually occurs on the right with a portion of the liver bulging cephalad.
Hilar Adenopathy
A differential of possible etiologies can be broken up into three different categories:
Inflammation (sarcoidosis, silicosis)
Neoplasm (lymphoma, metastases, bronchogenic carcinoma)
Infection (tuberculosis, histoplasmosis, infectious mononucleosis)
An important consideration to keep in mind is that since the pulmonary arteries also course through the same area, enlargement of these vessels may be confused with hilar adenopathy. Typically, lymphadenopathy has a more lumpy-bumpy appearance, while an enlarged pulmonary artery appears smooth.
Lung cancer
Adenocarcinoma – (35-50%) Peripheral, sometimes associated with scars, high incidence of early metastasis
Squamous Cell Carcinoma – (30%) Central, with hilar involvement, cavitation is common, slow growing
Small Cell - (15-20%) Central, cavitation is rare, hilar and mediastinal masses often the dominant feature, rapid growth and early metastases
Large cell – (10-15%) Peripheral, large, cavitation present
Bronchaveolar – (3%) Peripheral, rounded appearance, pneumonia-like infiltrate (air bronchograms), occasionally multifocal
Carcinoid – (less than 1%) Typically a well defined endobronchial lesion; nodal, liver and brain metastases may enhance densely (i.e. They may be hypervascular)
Tubes, Lines, Drains
Dobhoff - weighted end; specific for feeding
Central line
Peripheral IV
PICC Line
IJ Catheter
Pacemaker - SC tissue -
LVAD -
Pulmonary Artery Catheter -
Intra-aortic baloon pump -
Left ventricular assist device -
Endotracheal tubes (ET Tubes) or tracheostomy tubes
Cuffed conduits placed in the trachea either through the oropharynx or through a surgically created tracheostomy. These tubes maintain airway patency and allow for mechanical ventilation of patients with respiratory failure. A tracheostomy is generally performed in patients who are intubated for longer than 1-3 weeks or who have upper airway obstruction.
The carina can be assumed to be at the T4-T5 interspace, given that 95% of patients’ carinas project over the T5, T6, or T7 vertebral bodies.
The Dee method for approximating the position of the carina involves defining the aortic arch and then drawing a line inferomedially through the middle of the arch at a 45 degree angle to the midline. The intersection of the midline and the diagonal line is the most likely position of the carina.
Approximately, 10% of endotracheal tubes are malpositioned. The tube is more likely to enter the right main stem bronchus, due to its more vertical orientation, and reduce left lung ventilation, leading to collapse of the left lung. If the endotracheal tube enters into the bronchus intermedius, the right upper lobe can also collapse. Superiorly placed ET tubes may enter the pharynx or dislodge from the trachea into the esophagus causing filling of the stomach with air and, potentially, reflux of gastric contents. The glottis may also be damaged.
Major complications from endotracheal tubes are unusual. These include tracheal stenosis, tracheal ruputure, cord paralysis, cervical mediastinal emphysema, hematoma, and abscess formation.
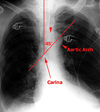
Thoracostomy tubes
Placed into the pleural space to evacuate either air or fluid. In the supine patient air collects anteriorly and fluid collects posteriorly. This dictates the proper positioning of the tube.
Thoracostomy tubes placed within fissures often cease to function when the lung surfaces become opposed. Also, incorrectly placed tubes for empyemas may delay drainage and result in loculation of the purulent fluid.
In order for thoracostomy tubes to function properly all of the fenestrations in the tube must be within the thoracic cavity. The last side-hole in a thoracostomy tube is indicated by a gap in the radiopaque line. If this interruption in the radiopaque line is not within the thoracic cavity or there is evidence of subcutaneous air, then the tube may not have been completely inserted.
Nasogastric and Feeding Tubes
Inserted through the nares and into the stomach. They are used for gastric decompression or feeding. Generally a chest x-ray is not necessary following the placement of a nasogastric tube.
Feeding tubes are generally placed into the proximal small bowel, as confirmed by an abdominal film. A chest x-ray may be obtained following the insertion of small-bore feeding tubes to rule out placement within the lung, which may have serious consequences. Also, patients who are status-post esophagectomy should receive a chest x-ray to evaluate the placement of any nasogastric tube.

Central Venous Pressure Monitors (line)
The intravascular volume status of critically ill patients is crucial to their management. A central venous pressure can be obtained directly via central vein catheters placed either through the subclavian veins or the internal jugular veins. Similarly, intravenous catheters may be used to infuse large volumes over longer periods of times with little chance of thrombosis.
Proper placement of central venous pressure monitors is necessary for accurate measurements. Ideally the catheter tip should lie between the most proximal venous valves of the subclavian or jugular veins and the right atrium.
Usually the last valve in the subclavian vein is at the level of the anterior portion of the first rib. Therefore, the tip should be medial to this point. (2.5 cm from where they join to form the brachiocephalic vein).
The most common locations for malpositioned catheters include the internal jugular vein, right atrium, and right ventricle. Arrhythmias or cardiac perforations may result from placement of lines within the heart.
Complications of central line placement may result in pneumothorax, occurring in as many as 6% of cases.
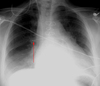
Swan-Ganz catheters (pulmonary capillary wedge pressure monitors)
Used to measure pulmonary wedge pressures. These catheters allow the intensivist to have an accurate measurement of the patient’s volume status and can help differentiate between cardiac and non-cardiac pulmonary edema.
Pulmonary capillary wedge pressure catheters (PCWP) are introduced percutaneously into the venous system. They are advanced through the right heart and into the pulmonary artery. A balloon at the end of the catheter is then inflated causing the tip of the catheter to be wedged into a branch of the pulmonary artery. The tip is “floated” to a distal pulmonary artery and wedged there. Once the tip is wedged, the balloon should be deflated. Once a reading is obtained, the tip is pulled back to the main pulmonary artery. The catheter tip should ideally be positioned no more distally than the proximal interlobar pulmonary arteries. A good rule of thumb is that the catheter tip should be within the mediastinal shadow. Placement more distally increases the chance of pulmonary infarction or vessel rupture.
Malpositioning of PCWP catheters is exceedingly common, found in approximately 25% of catheters placed. This may lead to false readings and an increased risk for complications. Complications of PCWP catheter placement include pneumothorax, pulmonary infarction, cardiac arrhythmias, pulmonary artery perforation, endocarditis, and sepsis.
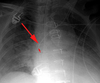
Intraaortic counterpulsation balloon pump (IACB)
Used to decrease afterload and increase cardiac perfusion in patients with cardiogenic shock. The IACB is synchronized with either the aortic pressures or the patient’s EKG to inflate during diastole and deflate during systole. Generally, the IACB is introduced percutaneously through the right femoral artery. Proper positioning of the IACB is critical to prevent occlusion of major vessels. Ideally the catheter should be in the region of the aortic isthmus or left main bronchus and above the origins of the celiac trunk and superior mesenteric artery. During systole the balloon may appear as a fusiform air (helium) containing radiolucency.

Transvenous Pacing Device
Patients in the ICU with bradyarrhythmias or heart block may require cardiac pacing. Transvenous pacers are introduced through the cephalic or subclavian vein into the apex of the right ventricle. Frontal and lateral projections are required to evaluate pacemaker placement. In the frontal view, the pacer tip should be at the apex with no sharp angulations throughout its length. On the lateral view, the tip should be imbedded within the cardiac trabeculae in such a way that it appears 3 to 4 mm beneath the epicardial fat stripe. A tip which appears beyond the epicardial fat stripe may have perforated the myocardium. Pacers placed within the coronary sinus will appear to be directed posteriorly on the lateral chest x-ray. The integrity of the pacer wire should be inspected along its entire length.
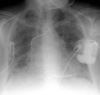
Pleural Effusion
Pleural effusions are accumulations of fluid within the pleural space. These fluids include blood, chyme, pus, transudates or exudates. In the ICU patient, pleural effusions are extremely common. In patients on medical services the most common cause is congestive heart failure, while up to two-thirds of patitient will develop pleural effusions following upper abdominal surgery. Patients undergoing thoracotomy or median sternotomy will also usually develop pleural effusions. Other causes of fluid accumulation in the intrapleural space include pulmonary embolism, neoplastic disease, subphrenic inflammatory processes (e.g. pancreatitis), pneumonia, trauma, and ascites.
On an upright film, an effusion will cause blunting on the lateral and if large enough, the posterior costophrenic sulci (meniscus sign). Sometimes a depression of the involved diaphragm will occur. A large effusion can lead to a mediastinal shift away from the effusion and opacify the hemothorax. In the supine film, an effusion will appear as a graded haze that is denser at the base. The vascular shadows can usually be seen through the effusion. An effusion in the supine view can veil the lung tissue, thicken fissure lines, and if large, cause a fluid cap over the apex.
Approximately 200 ml of fluid are needed to detect an effusion in the frontal film vs. approximately 75ml for the lateral.
Common causes are CHF, infection (parapneumonic), trauma, PE, tumor, autoimmune disease, and renal failure.
Dx: A lateral decubitis film is helpful in confirming an effusion in a bedridden patient as the fluid will layer out on the affected side (unless the fluid is loculated). Today, ultrasound is also a key component in the diagnosis. Ultrasound is also used to guide diagnostic aspiration of small effusions.

Pleural Effusions at the Apex
Fluid in the apex, in a supine patient, is more easily identified. A large pleural effusion may appear as a pleural cap with fluid occasionally collecting on the medial side, appearing as a widened mediastinum. Even with optimal radiographic techinique, small pleural effusions are difficult to identify in the supine patient.
>500 ml of fluid must accumulate before you expect to see changes in the supine patient’s chest x-ray
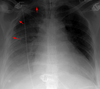
Pleural Effusions in the Erect Patient
In the erect patient fluid collects at the base of the chest. Costophrenic angle blunting and decreased visibility of the lower lobe vessels are commonly the result of pleural fluid pooling.
50-75ml of fluid must collect before costophrenic blunting is visible in the erect patient
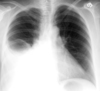
Lateral Decubitus Films for Pleural Effusions
Lateral decubitus films are an important way to comfirm the presence of pleural effusions. The film should be taken with the side of the patient suspected to have an effusion in the dependent position. The lateral decubitus position can also differentiate between loculated and free effusions. Loculations occur when the visceral and parietal pleura become partially adherent. They may require guided placement of chest tubes for adequate drainage.

Subpulmonic Effusions and Interlobar Pleural Effusions
Up to a liter of fluid may collect between the diaphragm and the lung without blunting of the costophrenic angle. Radiographically, subpulmonic effusions appear as a raised diaphragm with flattening and lateral displacement of the dome. The gastric bubble and splenic flexture of the colon show displacement inferiorly. The distance between the lung and the stomach bubble will exceed 2 cm in subpulmonary effusions. The lateral decubitus film can usually resolve any question of the presence of a subpulmonary effusion.
The diagnosis of interlobar effusion can often be challenging, especially in the presence of incomplete pleural fissures. A CT may be required to make the diagnosis. Another challenge can be differentiating between a loculated effusion in the minor fissure and right middle lobe atelectasis. An effusion appears as an homogenous density with biconvex edges and preservation of the minor fissure, while atelectasis appears as an inhomogenous density with concave margins and obliteration of both the right heart border and minor fissure.

Pericardial Effusion
Causes an enlarged heart shadow that is often globular shaped (transverse diameter is disproportionately increased). A “fat pad” sign, a soft tissue stripe wider than 2mm between the epicardial fat and the anterior mediastinal fat can be seen anterior to the heart on a lateral view. Serial films can be helpful in the diagnosis especially if rapid changes in the size of the heart shadow are observed. Approximately 400-500 ml of fluid must be in the pericardium to lead to a detectable change in the size of the heart shadow on PA CXR. Pericardial effusion can be definitively diagnosed with either echocardiography or CT. It can be critical to diagnose pericardial effusion because if it is acute it may lead to cardiac tamponade, and poor cardiac filling. In the postoperative patient it could be a sign of bleeding, necessitating a return to the OR.
Pericardial Effusions
Pericardial effusions are accumulations of fluid between the visceral (epicardium) and parietal pericardium. Several factors may lead to pericardial effusions including blockage of the lymphatic or venous systems by tumors, changes in osmostic or oncotic pressures due to metabolic diseases, or increased permeability of the pericardium due to inflammation. Blood in the pericardium (hemopericardium) may be an important clue to post operative bleeding. Effusions which do not raise the intrapericardial pressure more than 3 or 4 mmHg will not cause symptoms. The best evidence to determine if a pericardial effusion will become hemodynamically significant is to monitor how quickly it is accumulating. Best measured with echocardiography.
Radiographically, pericardial effusions appear as changes in the size and shape of the cardiac silhouette resulting a featureless, globular or “water bottle” shape. The pericardial fluid on an ICU film is generally not distinctly visible; instead it enlarges the cardiac shadow.

Pulmonary Edema
Occurs when fluid traverses capillary membranes and enters the alveolar space. It is the most common cause of decreased oxygenation in the ICU patient.
Three mechanisms lead to pulmonary edema. These are:
- Increased hydrostatic gradient
- Diminished oncotic pressure
- Increased capillary permeability due to endothelial injury
Any one or more often a combination of these mechanisms will cause fluid to enter the alveolar space.
Typically, the radiographic appearance of pulmonary edema includes one or more of the following: cephalization of pulmonary vessels, Kerley’s B lines peribronchial cuffing, bat wing pattern, patchy shawdowing with air bronchograms, and increased cardiac size. Generally, pulmonary edema is bilateral and may change rapidly. The radiographic appearance of the chest x-ray should also correlate with the central venous or pulmonary wedge pressure of the patient and clincal signs and symptoms of pulmonary edema.
Poor cardiac function will cause increased hydrostatic pressures in the pulmonary capillary bed resulting in cardiogenic pulmonary edema.
Non-cardiogenic pulmonary edema can result from volume overload due to renal failure, over hydration, or from diminished oncotic pressure in the liver failure patient, or from endothelial injury as in the patient with ARDS.
Presents with typical bilateral “batwing” increased pulmonary vasculature.

Cardogenic edema
Caused by increased hydrostatic pulmonary capillary pressure.
Cardiac edema is usually characterized by cardiac enlargment, pleural effusions, pulmonary vascular redistribution to the upper lobes, Kerley’s lines, peribronchial blurring, and basal edema.
Can show; cephalization of the pulmonary vessels, Kerley B lines or septal lines, peribronchial cuffing, “bat wing” pattern, patchy shadowing with air bronchograms, and increased cardiac size. Unilateral, miliary and lobar or lower zone edema are considered atypical patterns of cardiac pulmonary edema. A unilateral pattern may be caused by lying preferentially on one side. Unusual patterns of edema may be found in patients with COPD who have predominant upper lobe emphysema.
In a patient with CHF, the pulmonary capillary wedge pressure rises to the 12-18 mmHg range and the upper zone veins dilate and are equal in size or larger, termed cephalization.
With increasing PCWP, (18-24 mm. Hg.), interstitial edema occurs with the appearance of Kerley lines. Increased PCWP above this level is alveolar edema, often in a classic perihilar bat wing pattern of density. Pleural effusions also often occur.
Congestive Heart Failure
Cardogenic pulmonary edema is the result of left ventricular failure. Initially, increased filling volumes will increase contractility, as described by the Frank-Starling Curve. This mechanism though will fail if the ventricle is overstretched. The result is poor cardiac output and increased pulmonary venous hydrostatic pressures resulting in cardiogenic pulmonary edema. The combination of a weak heart and fluid overloading leads to congestive heart failure. Cardiac valvular disease, ischemic cardiomyopathy, renal failure and other causes may also lead to congestive heart failure. The chest radiograph plays an important role in distinguishing fluid overload or congestive failure causes before the onset of symptoms. Left-sided cardiac failure may be detected on a chest x-ray in 25-40% of patients in the event of an acute myocardial ischemia prior to clinical diagnosis. Under ideal situations, the chest film should be taken erect and in the PA view. Supine AP films reduce the viewers ability to detect cardiomegally and redistribution of pulmonary flow. Therefore, semierect and decubitus films are recommended in patients who may have new onset congestive heart failure.
As the left ventricle fails and begins to distend an enlarged cardiac silhouette is seen on x-ray, especially in patients with chronic CHF. This sign, though, is not specific; a pericardial effusion will also enlarge the cardiac silhouette. Also, AP films magnify the cardiac shadow making it difficult to determine actual cardiac enlargment. As pulmonary venous pressures rise pulmonary vessels are recruited in an attempt to normalize pressures. This phenomonan can be seen on chest x-ray as increased pulmonary vascularity with redistrubution to the apex. This signs is also compromised by the typical ICU portable film. Supine position of the patient will cause redistribution of pulmonary flow even in the abscence of CHF. The azygos vein may enlarge as a result of increased pressures transmitted to the venous system. This signs also depends on patient position. The more reliable signs of CHF in the ICU patient are alveolar or intersitial edema. Pleural effusions often accompany subacute or chronic cardiogenic pulmonary edema.

Noncardogenic pulmonary edema:
Caused by either altered capillary membrane permeability or decreased plasma oncotic pressure. NOT CARDIAC
Near-drowning, oxygen therapy, transfusion or trauma (fat embolism), CNS disorder, ARDS, aspiration, or altitude sickness, renal disorder or resuscitation, drugs, inhaled toxins, allergic alveolitis, contrast or contusion.
Cephalization
The initial phase of cardiogenic pulmonary edema is manifested as redistribution of the pulmonary veins. This is know as cephalization because the pulmonary veins of the superior zone dilate due to increased pressure. This diagnosis is made when the upper lobe vessels are equal to or larger in diameter than the lower lobe vessels. The diagnosis of cephalization is more difficult in the supine patient due to gravitational effects.

Kerley B lines (septal lines)
Horizontal lines less than 2cm long, commonly found in the lower zone periphery. These lines are the thickened, edematous interlobular septa.
Causes of Kerley B lines include; pulmonary edema, lymphangitis carcinomatosa and malignant lymphoma, viral and mycoplasmal pneumonia, interstital pulmonary fibrosis, pneumoconiosis, sarcoidosis.
They can be an evanescent sign on the CXR of a patient in and out of heart failure. Represent thickening of interlobular septa.
Remember that Kerley B lines will touch the pleura and blood vessels will not.

Interstitial Edema
Interstitial edema occurs as venous pressure rises into the 25-30 mmHg range. Interstitial edema as seen on the chest x-ray may in fact preceed clinical symptoms. This is testimony to the importance of the ICU chest film. Alveolar epithelial junctions are much tighter than endothelial cell junctions. Therefore, excess fluid accumulates in the intersitial space surrounding capillary walls first. Several signs are indicative of interstitial edema. The large pulmonary vessels may begin to lose definition and become hazy. Septal lines may begin to appear. Kerley’s A lines range from 5 to 10 cm in length and extend from the hila toward the periphery in a straight or slightly curved course. They represent fluid in the deep septa and lymphatics, usually in the upper lobes. Kerley’s B lines are shorter thin lines (1.5 to 2.0 cm in length) and are seen in the periphery of the lower lung, extending to the pleura (see below). These represent interlobular septal thickening. The chest x-ray in interstitial edema may take on a diffuse reticular pattern resembling widespread interstitial fibrosis. Peribronchial cuffing represents interstitial edema and appears as very thick bronchial walls.

Alveolar Edema
Alveolar edema occurs when the pulmonary venous pressure exceeds 30 mmHg. Therefore, the signs of interstitial edema are present in patients who have progressed to alveolar edema. Classically, alveolar edema appears as bilateral opacities that extend in a fan shape outward from the hilum in a “bat wing” pattern. As the edema worsens, the opacities become increasingly homogenous. These water-density opacities may contrast with air-filled bronchi which, in normally aerated parenchyma are invisible. The visible appearance of previously imperceptible bronchi is known as air-bronchograms.

Atypical patterns of pulmonary edema
Can represent a challenge for the radiologist. Pulmonary edema may be unilateral, lobar, miliary, or restricted to the lower zones of the lung. Pulmonary edema may assume any asymmetric or unusual distribution. Although gravitiy as been implicate as the culprit many other theories have been devised to explain the bizarre patterns of pulmonary edema noted. Miliary edema is often considered a normal transitory phase in the development of full scale edema. Lobar or lower zone dema is found in patient suffering from chronic obstructive pulmonary disease with predominate upper lobe emphysema.
One method of differentiating pulmonary edema from other causes of lung opacities is the gravitational shift test. The patient is kept in the supine position for two hours before a chest film is taken. Then the patient is left in the decubitis position with the suspicious hemithorax in the independent position for 2 to 3 hours before a second film is taken. In 85% of patients with pulmonary edema there is a shift in the opacity as opposed to 80% of patient without pulmonary edema who had no shift.
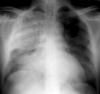
Adult respiratory distress syndrome (ARDS)
ARDS is a term used to describe a constellation of clinical and radiographic signs and symptoms reflecting pulmonary edema in the absence of elevated pulmonary venous pressures. ARDS is relatively common in the ICU population and is associated with high mortality (~50%). The syndrome results from a variety of causes, including sepsis or pulmonary infection, severe trauma, and aspiration of gastric contents, all of which together account for 80% of cases. Whatever the initial cause, all share activation of the complement pathway with damage to the alveolar capillary endothelium, increased vascular permeability, and subsequent development of first interstitial and then alveolar pulmonary edema. Clinically, there is severe respiratory distress characterized by marked hypoxia that responds poorly even to administration of high concentrations of oxygen. Pulmonary capillary wedge pressure is usually normal. Decreased surfactant production leads to poor lung compliance and atelectasis that results in an intrapulmonary shunt with perfusion but no effective ventilation. Positive End Expiratory Pressure (PEEP) can help to decrease atelectasis, shunting while improving oxygenation. Patients surviving the syndrome may progress to pulmonary fibrosis or have no sequelae. The longer and more severe the ARDS, the more likely are long term consequences. Other factors may also contribute, such as age and preexisting COPD.

ARDS versus Congestive Heart Failure
While it is not always easy, it is often possible to radiographically distinguish between pulmonary edema caused by congestive heart failure (CHF) and ARDS. Indeed, both may coexist. Although both entities may share the x-ray finding of bilateral airspace opacification or “white out”, ARDS is not associated with cardiomegaly or with cephalization of pulmonary vasculature. However, cephalization may not be visible in the midst of “white out”and CHF can exist without cardiomegaly. Both of these findings may be difficult to discern in the supine patient. The patient with ARDs could also have preexistant cardiomegaly or be fluid overloaded because of sepsis.
Features that are helpful in distinguishing CHF from ARDS include the following: While cardiogenic pulmonary edema typically begins centrally in the bilateral perihilar areas, ARDS usually causes more uniform opacification. Pleural effusions are not typical of ARDS but often present in CHF. Kerley B lines are common in CHF but not in ARDS, while air bronchograms can be found in both.
Temporally, radiographic abnormalities usually closely parallel cardiogenic pulmonary edema, while the chest radiograph in ARDS may remain unremarkable for up to twelve hours and usually stabilize after the first thirty-six hours. While radiographic findings in cardiogenic edema may clear rapidly, ARDS typically clears slowly. Unlike cardiogenic edema, which, once resolved, does not leave behind permanent pulmonary changes, a percentage of ARDS cases will result in some degree of permanent pulmonary fibrosis, characterized by increased intersitital markings depending on the severity and length of time the patient was in ARDS.
Atelectasis
Atelectasis is a term used to describe reduced inflation in part of the lung.
Atelectasis in ICU patients occurs most frequently in the left lower lobe, presumably due to compression of the lower lobe bronchus by the heart, in the supine patient. Contributing to this tendency is the relatively greater difficulty of blind suctioning of the left lower lobe. The etiology of atelectasis includes any process which reduces aveolar ventilation including general anesthesia, splinting from pain following surgery, or bronchial obstruction by mucus plugging. Mobilization of secretions may be inhibited by inflammatory lung disease, edema, or tracheal intubation. The final result is reduced alveolar distention resulting in decreased surfactant production which propagates the ateletasis further. Usually atelectasis is more extensive than is suggested by the radiograph. Extensive alveolar hypoventilation may result in an effective right to left shunt and subsequent hypoxia. Atelectasis is reversible and preventable with the use of hyperventilation and incentive spirometry especially in the post-operative period.
Is most often caused by an endobronchial lesion, such as mucus plug or tumor. It can also be caused by extrinsic compression centrally by a mass such as lymph nodes or peripheral compression by pleural effusion. An unusual type of atelectasis is cicatricial and is secondary to scarring, TB, or status post radiation.
Atelectasis is almost always associated with a linear increased density on chest x-ray. The apex tends to be at the hilum. The density is associated with volume loss. Some indirect signs of volume loss include vascular crowding or fissural, tracheal, or mediastinal shift, towards the collapse. There may be compensatory hyperinflation of adjacent lobes, or hilar elevation (upper lobe collapse) or depression (lower lobe collapse). Segmental and subsegmental collapse may show linear, curvilinear, wedge shaped opacities. This is most often associated with post-op patients and those with massive hepatosplenomegaly or ascites .
Radiographically, atelectasis may vary from complete lung collapse to relatively normal-appearing lungs. For example, acute mucus plugging may cause only a slight diffuse reduction in lobar or lung volume without visible opacity. Nevertheless, the physiologic effects can be significant. In the so called mucus plugging syndrome, the association of sudden hypoxia with a normal or quasi-normal chest radiograph can lead to the suspicion of a pulmonary embolus. Mild atelectasis usually takes the form of minimal basilar shadowing or linear streaks (subsegmental or “discoid” atelectasis) and may not be physiologically significant. Atelectasis may also appear similar to pulmonary consolidation (dense opacification of all or a portion of a lung due to filling of air spaces by abnormal material), making it difficult to distinguish from pneumonia or other causes of consolidation. The distinction between atelectasis and other causes of consolidation is important, and certain clues exist to aid in making that determination. Atelectasis will often respond to increased ventilation, while pneumonia, for example, will not. Crowding of vessels, shifting of structures such as interlobar fissures towards areas of lung volume loss and elevation of the hemidiaphragm suggests atelectasis. Another key for distinguishing between atelectasis and consolidation is recognition of the typical patterns that each pulmonary lobe follows when collapsing.
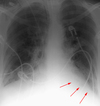
Right Upper Lobe Atelectasis
Right upper lobe atelectasis is easily detected as the lobe migrates superomedially toward the apex and mediastinum. The minor fissure elevates and the inferior border of the collapsed lobe is a well demarcated curvilinear border arcing from the hilum towards the apex with inferior concavity. Due to reactive hyperaeration of the lower lobe, the lower lobe artery will often be displaced superiorly on a frontal view.

Left Upper Lobe Atelectasis
The left lung lacks a middle lobe and therefore a minor fissure, so left upper lobe atelectasis presents a different picture from that of the right upper lobe collapse. The result is predominantly anterior shift of the upper lobe in left upper lobe collapse, with loss of the left upper cardiac border. The expanded lower lobe will migrate to a location both superior and posterior to the upper lobe in order to occupy the vacated space. As the lower lobe expands, the lower lobe artery shifts superiorly. The left mainstem bronchus also rotates to a nearly horizontal position.

Right Middle Lobe Atelectasis
Right middle lobe atelectasis may cause minimal changes on the frontal chest film. A loss of definition of the right heart border is the key finding. Right middle lobe collapse is usually more easily seen in the lateral view. The horizontal and lower portion of the major fissures start to approximate with increasing opacity leading to a wedge of opacity pointing to the hilum. Like other cases of atelectasis, this collapse may by confused with right middle lobe pneumonia.

Left Lower Lobe Atelectasis
Atelectasis of either the right or left lower lobe presents a similar appearance. Silhouetting of the corresponding hemidiaphragm, crowding of vessels, and air bronchograms are standard, and silhouetting of descending aorta is seen on the left. It is important to remember that these findings are all nonspecific, often occuring in cases of consolidation, as well. A substantially collapsed lower lobe will usually show as a triangular opacity situated posteromedially against the mediastinum.
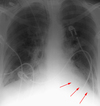
Right Lower Lobe Atelectasis
Silhouetting of the right hemidiaphragm and air bronchograms are common signs of right lower lobe atelectasis. Right lower lobe atelectasis can be distinguished from right middle lobe atelectasis by the persistance of the right heart border.

Aspiration Syndromes
ICU patients are at risk for aspiration pneumonitis. Reduced consciousness, neuromuscular disorders, and intratracheal or intraesophageal devices all are factors which may predispose patients to aspiration by compromising the patient’s airway defense mechanisms.The effects of aspiration are determined by volume of the aspirate and the nature of the aspirated material. These determine the extent and severity of any inflammatory response. Chemical irritants may be acid, alkali or paticulate in nature depending on gastric contents.
Aspiration of gastric acid is also known as Mendelson’s Syndrome, it is the most common type of aspiration. The degree of irritation to the lung is directly dependent on the acidity and volume of the aspirated fluid. The lung responds to pH < 2.5 with severe bronchospasm and the release of inflammatory mediators. The initial result is a chemical pulmonary edema. Secondary infection may or may not result. The clinical manifestations occur within minutes of the event and include cough, dyspnea, wheezing and diffuse crackles. Fever and an elevated white count will occur in the majority of patients. The consequences of aspiration range from shock to uncomplicated resolution of the initial event. The chest film in patients that progress to pneumonitis will reveal pulmonary consolidation within the first two days. The consolidation is usually perihilar and bilateral, though asymmetric. The radiographic findings begin to stablize or resolve by the third day. Some patients’ radiographs will show worsening of the consolidation as well as findings associated with pneumonia, including pleural effusions and abscess formation. Aspiration may also cause ARDS.



