Chemical Names First Flashcards
1
Q
Sulfuric Acid
A
H2SO4
2
Q
sulfurous acid
A
H2SO3
3
Q
nitric acid
A
HNO3
4
Q
nitrous acid
A
HNO2
5
Q
hydrochloric acid
A
HCl
6
Q
hydrobromic acid
A
HBr
7
Q
hypochlorous acid
A
HClO
8
Q
chlorous acid
A
HClO2
9
Q
chloric acid
A
HClO3
10
Q
perchloric acid
A
HClO4
11
Q
hypobromous acid
A
HBrO
12
Q
bromous acid
A
HBrO2
13
Q
bromic acid
A
HBrO3
14
Q
perbromic acid
A
HBrO4
15
Q
hydrogen cyanide (prussic acid)
A
HCN
16
Q
metaphosphoric acid
A
(HPO3)n
17
Q
orthophosphoric acid
A
H3PO4
18
Q
boric acid
A
H3BO3
19
Q
carbonic acid
A
H2CO3/ OC(OH)2
20
Q
ammonia
A
NH3
21
Q
hydrazine
A
N2H4
22
Q
hydroxylamine
A
HO-NH2
23
Q
hydrogen peroxide
A
H2O2
24
Q
superoxide anion
A
O2-
25
pyrophosphate anion
P2O74- (aka PPi)
26
hydrogen sulfide
H2S
27
carbon monoxide
CO
28
carbon dioxide
CO2
29
nitrous oxide
N2O
30
hydrogen cyanide (structure)

31
carbonic acid (structure)

32
carbon monoxide (structure)

33
carbon dioxide (structure)

34
benzene (structure)
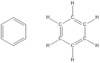
35
napthalene (structure)

36
phenanthrene (structure)

37
pyrolle (structure)

38
sulfur dioxide
SO2
39
sulfur trioxide
SO3
40
hydroxyapatite
Ca5(PO4)3(OH)
41
fluoroapatite
Ca5(PO4)3F
42
ferrous ammonium sulfate
(NH4)2 Fe(SO4)2\*6H2O
43
# structure:
thiophene (structure)

44
# structure:
furane (structure)

45
# structure:
thiazole (structure)

46
# structure:
oxazole

47
imidazole
*\*N's are further away than pyrazole*

48
# structure:
pyrazole

49
# structure:
pyrene

50
# structure:
pyran

51
# structure:
pyrazine

52
# structure:
pyrimidine

53
purine

54
indole

55
pteridine

56
acridine

57
methanol

58
ethanol

59
propanol

60
isopropanol (isopropyl alcohol)

61
n-butanol

62
ethylene glycol

63
glycerol

64
inositol

65
phenol

66
diethylether

67
formaldehyde

68
acetaldehyde

69
acetone

70
mercaptoethanol

71
aniline

72
urea

73
guanidine

74
formic acid

75
acetic acid

76
propionic acid

77
butyric acid

78
valeric acid

79
caproic acid

80
oxalic acid

81
malonic acid

82
succinic acid

83
glutaric acid

84
maleic acid
cis side compared to fumaric acid
differs from malic acid in that it has a double bond and no OH
("e" as in "pie" as in "pi" bond)

85
fumaric acid
trans of maleic acid

86
lactic acid

87
b-hydroxybutyric acid

88
pyruvic acid

89
acetoacetic acid

90
citric acid

91
cis-aconitic acid

92
isocitric acid

93
a-ketoglutaric acid

94
malic acid
\*no double bond as in maleic acid\*
\*\*dehydrogenation makes oxaloacetic acid\*\*

95
oxaloacetic acid

96
lactone molecules

97
ether



