Chapter 4.1 - Atomic Theory & Bonding Flashcards
(26 cards)
What are atoms?
The smallest particle of any element
What are subatomic particles?
The particles that make up an atom
What is nuclear charge?
The electric charge on the nucleus
What are the 3 subatomic particles?
Protons, neutrons, and electrons
How do you find the number of neutrons in an atom?
Atomic mass - number of protons = number of neutrons
What is the atomic number?
The number of protons
What are Bohr diagrams?
Diagrams that show the arrangement of subatomic particles
What is a stable octet?
When an atom’s or ion’s second shell is full
What is a valence shell?
The outermost shell
What are valence electrons?
The electrons in the outermost shell
What is a lone pair?
A pair of valence electrons that are not shared with another atom
What is an ionic compound?
A compound that contains a positive ion (metal) and a negative ion (non-metal)
What is ionic bonding?
The complete transfer of valence electron(s) between atoms
What is a cation?
Forms when a metal atom loses electrons
What is a multivalent metal?
A metal with more than one ion charge
What is an anion?
Forms when non-metal atoms gain electrons
What is a covalent compound?
Compound that contains 2 or more non-metals
What is covalent bonding?
The stable balance of attractive and repulsive forces between atoms when they share electrons
What is a covalent bond?
A chemical bond that involves the sharing of electron pairs between atoms
What are Lewis diagrams?
Diagrams that illustrate chemical bonding by showing only an atom’s valence electrons and its chemical symbol
Name the letters:

A: Atomic number
B: Ion charges
C: Chemical symbol
D: Element name
E: Atomic mass
Practice: What element is this?

Fluorine
Practice: What element is this?

Nitrogen
Practice: What element is this?
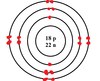
Argon


