Chapter 31: Signaling and Cancer Flashcards
Cancer Affects Everyone, Directly or Indirectly
- It was estimated for 2013 that in the USA that there would be ~1,660,290 new diagnoses of cancer and that ~580,350 will die of it = 35%.
- The lifetime probability for being diagnosed with invasive cancer is 48% in white males and 38% in white females based on statistics gathered from 2006 to 2008.
- One in five of us will die of cancer.
- Minnesota has an incidence rate of 573/100,000 compared to the national average of 553/100,000

Cancer
- Increased cell proliferation
- Decreased response to apoptotic signals
Normal Cells Have Many Ways to Control Cell Proliferation
- Contact inhibition: cells don’t pile up on each other
- A requirement for mitogen and growth factor stimulation to synthesize the D cyclins
- Replicative senescence: cells are alive but are unable to divide due to an aging process associated with telomere shortening
- Checks to prevent overstimulation by mitogens
- Desensitization to mitogen signaling
- Activation of p53
Checks to Prevent Overstimulation: Activation of p53
- By unknown mechanisms, excessive mitogen stimulation leads to production of more Arf in normal cells.
- Arf sequesters the Mdm2 E3 ubiquitin ligase so that it can not ubiquitinate p53.
- p53 is stabilized and can cause cell cycle arrest or apoptosis
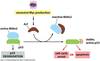
Conversion of a Proto-Oncogene to an Oncogene Can Cause:
- increased levels of the proto-oncoprotein, which will increase signaling inappropriately
- Ex: Increased amounts of RTKs will make the cells hypersensitive to otherwise-limiting amounts of GF
- alterations in signaling proteins such as in RTKs that make signaling ligand-independent
- Ex: Mutations that delete the external domain of RTKs or change their dimerization properties so that activation of the RTK is ligand-independent signaling
- new fusion proteins to be generated that have hyperactive signaling properties
A Gene Fusion Can Lead to Constitutively Dimerized Receptors
- The genes encoding RTKs sometimes become fused to unrelated genes that encode proteins that normally dimerize or form oligomers without a ligand. Thus, the RTKs are dragged together by their fusion partners. This causes ligand-independent receptor signaling.

Fusion of Other Proto-Oncoproteins Can Also Cause Cancer: The BCR-Able Gene Fusion
- A reciprocal translocation between the 9 and 22 chromosomes creates the Philadelphia chromosome. Transcription generates the BCR-ABL fusion protein from the BCR and Able kinase genes.
- The function of BCR is unknown but Abl is a membrane-associated tyrosine kinase (like Src).
- The tyrosine kinase activity of the BCR-ABL fusion protein is always active, inappropriately signaling cells to proliferate.
- When this fusion protein occurs in bone marrow, depending on the actual chromosomal breakpoint, it leads to chronic myeloid leukemia (CML), acute lymphoblastic leukemia (ALL), or chronic neutrophilic leukemia

The BCR-Abl Kinase Is Constitutively Active and Inappropriately Phosphorylates Many Signaling Proteins
- For example, the JAK/STAT pathway gets activated even without cytokines, which is why the leukemias occur.
- Specific tyrosine kinase inhibitors have been developed (Gleevec™ = Imatinib) that initially cause disappearance of the disease in 80% of patients.
- The catch? In about 5% of the patients, the kinase becomes resistant, mostly due to a point mutation in the active site.
- First cancer drug targeted to a signaling protein unique to cancer cells!

Studies of Rare Hereditary Cancer Syndromes First Identified Tumor Suppressor Genes
In the hereditary form, all cells in the body lack function of one of the two genes that encode Rb. Tumors occur when the normal allele is inactivated or lost by a somatic event. In the nonhereditary form, both alleles have to be lost or mutated.

Epigenetic Inheritance
- The heritable and reversible modifications that affect gene expression (transcription) and genome stability without changing the nucleotide sequence.
- DNA methylation (HATs and HDACs)
- Histone modifications
- Long noncoding RNAs (ex: X-chromosome inactivation in females)
- This is the cellular ‘memory’ of that cell’s experiences that affect chromatin structure.
- The memory is typically established and erased (reversed) by enzymes.
- Epigenetic events are much more frequent than genetic events (nucleotide changes).
DNA Methylation of CpG Islands Typically Leads to…
- Gene Silencing
- It is estimated that most tissue-specific gene silencing is the result of DNA methylation at promoters
- Can be inherited
How Do Both Hypermethylation and
Hypomethylation Occur in Cancer?
hypermethylation of promoters for tumor suppressor genes silences them and hypomethylation of promoters for oncogenes activates them

Comparison of Oncogenes and Tumor Suppressor Genes
- Oncogenes
- Dominant: Mutation in one of the alleles is sufficient
- Gain of function of a protein that signals cell proliferation or growth
- Mutation arises in somatic cells, not inherited
- Some tissue preference
- Tumor Suppressor Genes
- Recessive: Both alleles must be affected (exception, p53)
- Loss of function of a protein that inhibits cell proliferation or growth or repairs DNA
- Mutations present in germ cell (inherited) OR in somatic cells
- Often strong tissue preference
p53 Pathway
regulates responses to stress and to DNA damage
Rb Pathway
Initiation of the cell division cycle
RTK/Ras/PI 3K Pathway
transmits signals for cell growth from the exterior of the cell to the nucleus
Mutations in TP53 or Other Related Genes Are Found in Most Cancers- More Than in any Other Gene. Why?
- The functions of p53 are critical: it serves as an ‘antenna’, alerting the cell to any dangerous situations
- Promotes cell cycle arrest when DNA is damaged
- Triggers DNA repair mechanisms
- Initiates apoptosis when damage is too severe
- Blocks angiogenesis
- Blocks excessive mitogen signaling
- p53 is not required for normal development, so mutations in it are not lethal
Because p53 Functions as a Tetramer, Mutation of Only One Allele of TP53 Can Permit Cancer
- Mutations in p53 are typically dominant negative mutations as only 1 allele needs to be affected to permit cancer if the mutated protein can participate in tetramer formation.
- Ex: Li-Fraumeni syndrome. Individuals with this disease develop many tumors early in life. This is transmitted as an autosomal dominant disease.
Most Cancers Derive From a Single Abnormal Cell
In this example, the tumor has only chromosomes from the mom, suggesting clonal origin.

A Single Mutation Is Not Enough to Cause Cancer
- ~1016 cell divisions occur in humans in a lifetime
- Spontaneous mutations in a carcinogen-free environment occur about 10-6 per gene per cell division (based on knowledge of the accuracy of DNA replication and repair)
- In a human lifespan, each gene is likely to have been mutated 1010 times.
- Why isn’t cancer more frequent?
- Multiple genetic (at least 2) events are usually needed
Evidence for the Theory That Cancer Is a Multi-Hit Event Arises Mostly from Epidemiology Studies
- Cancer increases exponentially as a function of age.
- If a single mutation were responsible with a fixed probability of occurring any year, then this graph would be linear.
- Instead, this graph suggests there is a progressive accumulation of random mutations over time.
- Therefore, non-familial cancer is (mostly) a disease of age.

Sequential Progression of Tumorigenesis Due to Multiple Lesions Makes Sense for Slow Growing Solid Tumors, But it Does Not Explain a Number of Observations
- What about rapidly growing cells (ex. skin, blood cell) where there isn’t the time for multiple genetic hits?
- Leukemias and lymphomas are very frequent yet no single cells, except stem cells, are around long enough to get multiple hits
- What about tumor heterogeneity?
- Tumors are only homogeneous (clonal) early in progression but then become heterogeneous
- Why do some recurrent cancers have the same heterogeneity as the original cancer?
- Why are only a few cells (1 - 3%) in a tumor actually tumorigenic?
Few Cells in Tumors Are Tumorigenic
- Less than 1% chance that any randomly chosen cell from a tumor (including solid tumors) can cause a new tumor when transferred. How can this be if the cells are clonal?
- New cell sorting technologies have shown that is because only a small % of them have the properties associated with tumor propagation
- This is consistent with the number of stem cells/CSCs that are thought to be present in a tissue
- Considerable effort is now being exerted to identify CSC ‘markers’ so that the CSCs can be isolated and studied
Current Therapies May Promote
CSC Survival and Propagation
- SCs and CSCs generally replicate very, very infrequently. (A single cell cycle is fast, but they don’t replicate often.) Radiation and many chemotherapies target frequently dividing cells. This likely just ‘debulks’ the tumor by getting rid of the cells that are not really tumorigenic and increasing the percentage of CSCs.
- In addition, SCs and CSCs are naturally resistant to chemotherapeutic agents as they have ABC transporters that pump out the drugs. Thus, the CSCs would live as they are resistant to the drugs and the non-tumorigenic cells die, again leaving a larger percentage of CSCs.
- One hypothesis is that recurrent cancers (cancers that come back) are largely the consequence of CSCs slowly repopulating the area.



