Chapter 13: Alkenes Flashcards
- Describe the structure of alkenes.
- What is the general formula of aliphatic alkenes that contain one doudble bond?
- Describe other alkenes. Which of these follow the general formula of aliphatic alkenes that contain one double bond?
- Alkenes are unsaturated hydrocarbons; alkene molecules contain at leat one carbon-to-carbon double bond in their structure.
- Aliphatic alkenes that contain one double bond have the general formula CnH2n.
- Alkenes can be branched, can contain more than one double bond, or be cyclic. Branched alkenes obey the general formula CnH2n but cyclic alkenes and alkenes with more than one double bond do not.
Describe the types of bonds that a carbon atom of a double bond in an alkene forms.
- For each carbon atom of the double bond, three of the four electrons are used in three σ-bonds: one to the other carbon atom of the double bond, two to the other two atoms (carbon or hydrogen).
- The leftover electron on each carbon atom of the double bond is in a p-orbital. A π-bond is formed by the sideways overlap of two p-orbitals, one from each carbon atom of the double bond.
- The π-electron density is concentrated above and below the line joining the nuclei of the bonding atoms.
Describe how the π-bond contributes to the geometry of alkenes.
The π-bond locks the two carbon atoms in position and prevents them from rotating around the double bond. This makes the geometry of the alkenes different from that of the alkanes, where rotation is possible around every atom.
Illustrate the formation of the C=C bond in ethene.

Describe and explain the shape around each of the carbon atoms in the double bond.
The shape around each of the carbon atoms in the double bond is trigonal planar because:
- there are three regions of electron density around each of the carbon atoms
- the three regions repel each other as far as possible, so the bond angle around each carbon atom is 120˚
- all of the atoms are in the same plane.
What are stereoisomers?
- Stereoisomers have the same structural formula but a different arrangement of the atoms in space. There are two types of stereoisomerism: E/Z isomerism and optical isomerism.
- E/Z isomerism only occurs in compounds with a C=C double bond, whereas optical isomerism can occur in a much wider range of compounds, including alkanes wih no functional groups.
- Why does stereoisomerism around double bonds arise?
- What is the reason for this rigidity?
- Stereoisomerism around double bonds arises because rotation about the double bond is restricted and the groups attached to each carbon atom are therefore fixed relative to each other.
- The reason for rigidity is the position of the π-bond’s electron density above a belwo the place of the σ bond.
- Describe how a molecule is determined as having E/Z isomerism.
A molecule has E/Z isomerism if the molecule satisfies both the following conditions:
- has a C=C double bond
- has two different groups attached to each carbon atom of the double bond.

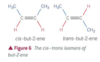
What is meant by cis-trans isomerism? Give an example.
- Cis-trans isomerism is used to describe a special case of E/Z isomerism in which one of the attached groups on each carbon atom of the double bond must be hydrogen.
- In cis-trans isomerism:
- the cis isomer is the Z isomer (hydrogen atoms on each carbon on same side of molecule)
- the trans isomer is the E isomer (hydrogen atoms diagonally opposite each other).
- For example, but-2-ene is a molecule with E/Z isomers that can also be describe dwith cis-trans nomenclature.
What is Cahn-Ingold-Prelog nomenclature used for?
Describe the process of using the Cahn-Ingold-Prelog rules.
- Cahn-Ingold-Prelog nomenclature is used to name E/Z isomers that do not show cis-trans properties.
- The CIP system assigns priorities to the atoms attached to each carbon atom in a double bond based on their atomic number.
- The higher the atomic number, the higher the priority.
- If the groups of higher priority (higher atomic number) are on the same side of the double bond, the compound is the Z isomer.
- If the groups of higher priority are diagonally placed across the double bond, the compound is the E isomer.
EXAM NOTE: If the two atoms attached to a carbon atom in the double bond are the same, then you will need to find the first point of difference.

Describe the basis for the mechanism of the addition reaction of alkenes to form saturated compounds.
The mechanism reaction for the addition reaction of alkenes to form saturated compounds is called electrophilic addition.
- The double bond in an alkene represents a region of high electron density because of the presence of the π-electrons.
- The high electron density of the π-electrons attracts electrophiles.
- An electrophile is an atom or group of atoms that is attracted to an electron-rich centre and accepts an electron pair. An electrophile is usually a positive ion or a molecule containing an atom with a partial positive (𝛿+) charge.
Using displayed formula, give the equation for the addition reaction between hydrogen bromide and but-2-ene.

Describe and illustrate the mechanism for the addition reaction between but-2-ene and hydrogen bromide.
- Bromine is more electronegative than hydrogen, so hydrogen bromide is polar and contains the dipole
H𝛿+–Br𝛿–. - The electron pair in the π-bond is attracted to the partially positive hydrogen atom, causing the double bond to break.
- A bond forms between the hydrogen atom of the H–Br molecule and a carbon atom that was part of the double bond.
- Instantaneously, the H–Br bond breaks by heterolyric fission, with the electron pair going to the bromine atom.
- A bromide ion (Br–) and a carbocation are formed. A carbocation contains a positively charged carbon atom.
- In the final step the Br– ion reacts with the carbocation to form the addition product.

Using displayed formulae, give the equation for the additional reaction between propene and bromine.

Describe the polarisation of the Br–Br bond in bromine in the addition reaction of propene and bromine.
Bromine is a non-polar molecule. When bromine approaches an alkene, the π-electrons interact with the electrons in the Br–Br bond.
This interaction causes polarisation of the Br–Br bond, with one end of the molecule becoming Br𝛿+ and the other end f the molecule becoming Br𝛿–. This is known as an induced dipole.

Describe and illsutrate the mechanism for the electrophilic addition reaction of propene with bromine.
- The bromine approaches the alkene, which causes polarisation of the Br–Br bond.
- The electron pair in the π-bond is attracted to the Br𝛿+ end of the molecule, causing the double bond to break.
- A bond has now been formed between one of the carbon atoms from the double bond and a bromine atom.
- The Br–Br bond breaks by heterolytic fission, with the electron pair going to the Br𝛿– end of the molecule.
- A bromide ion (Br–) and a carbocation are formed.
- In the final stage of the reaction mechanism, the Br– ion reacts with the carbocation to form the addition product of the reaction.

State Markownikoff’s rule.
- The rule states that with the addition of a hydrogen halide to an unsymmetrical alkene, the hydrogen becomes attached to the carbon with more hydrogen substituents, and the halide group becomes attached to the carbon with more carbon substituents.
In the addition reaction of an alkene with a hydrogen halide, describe how the major product is determined.
- Carbocations are classified by the number of alkyl groups attached to the positively charged carbon atom. Tertiary carbocations are the most stable, and primary carbocations are the least stable.
- Carbocation stability is linked to the electron-donating ability of alkyl groups. Each alkyl group donates and pushes electrons towards the positive charge of the carbocation. The positive charge is spread over the alkyl groups. The more alkyl groups attached to the positively-charged carbon atom, the more the charge is spread out, making the ion more stable.
- Therefore, tertiary carbocations are more stable than secondary carbocations, which are more stable than primary carbocations.
- Addition of a hydrogen halide to an unsymmetrical alkene forms the major product via the most stable carbocation possible.

Explain which of the two isomers formed when propene reacts with a hydrogen halide is the major product.
- Two possible carbocations are formed when propene reacts to a hydrogen halide: a primary carbocation and a secondary carbocation.
- Markownikoff’s rule states that the hydrogen in a hydrogen halide becomes attached to the carbon with more hydrogen substituents. This is the carbon at the end of the chain.
- A positive charge is left on the middle carbon so this is a secondary carbocation (as there are two alkyl groups attached to the middle carbon).
- The Br– ion reacts with the secondary carbocation to form 2-bromopropane. This is the major product.
- What are polymers?
- Where is addition polymerisation used?
- How is industrial polymerisation carried out?
- How are synthetic polymers named?
- Show the general balanced equation for an addition polymerisation reaction.
- Polymers are extremely large molecules formed from many thousands of repeat units of smaller molecules known as monomers.
- Unsaturated alkene molecules undergo addition polymerisation to produce long saturated chains containing no double bonds.
- Industrial polymerisation is carried out at high temperature and high pressure using catalysts.
- Synthetic polymers are usually named after the monomer that reacts to form their giant molecules, prefixed by ‘poly’.
- Image below.

- What is a repeat unit?
- How is the repeat unit shown?
- What does n denote in an equation for addition polymerisation?
- A repeat unit is the specific arrangement of atoms in the polymer molecule that repeats over and over again.
- The repeat unit is always written in square brackets.
- The letter n signifies many units. It is placed before the monomer unit to show that there is a large number of monomer units and after the square brackets to show that there is a large number of repeats.
Addition polymers are usually made from one type of monomer unit.
How is the polymer poly(ethene) made? Explain.
- The polymer poly(ethene) is made by heating a large number of ethene monomers at high pressures.

What is poly(chloroethene)?
- Poly(chloroethene) — also known as poly(vinyl chloride) and PVC — is a polymer prepared from the monomer unit chloroethene (vinyl chloride).
- It can be prepared to make a polymer that is flexible or rigid.

Give the monomer units, displayed formula, and uses of propene, phenylethene and tetrafluoroethene.

Expand on the following environmental concern headings:
- PVC recycling
- waste polymer fuel
- feedstock recycling
-
PVC Recycling. Disposal and recycling of PVC is hazardous due to high chlorine content and additives. Dumping PVC in landfill not suitable and releases pollutants (hydrogen chloride).
New tech uses solvents to dissolve polymer. High-grade PVS is then recovered by precipitation from the solvent, and the solvent is used again. - Waste Polymer Fuel. Polymers derived from petroleum or natural gas have a high stored energy values. These polymers can be incinerated to produce heat, generating steam to drive a turbine producing electricity.
- Feedstock Recycling. Chemical and thermal processes that can reclain monomers, gases, or oil from waste polymers. These can be used as raw materials for production of new polymers. FR is able to handle unsorted and unwashed polymers.
Describe biodegradable and photodegradable polymers.



