Chapter 120 Adrenal Glands Flashcards
At what level does left and right adrenal gland site
R at T13
L at L2
What emvryonic tissue is adrenal cortex derived from? And adrenal medulla?
Cortex derived from mesoderm
Medulla derived from neural crest ectoderm (migrates into centre of mesenchymal ball)
From which vessels does the adrenal gland receive its arterial supply?
Arterial supply
- Aorta
- Phrenicoabdominal artery
- Renal artery
- Cranial abdominal artery
Venous drainage
- Single adrenal vein (into cava on R, into renal vein on L)

WHat are the three layers of the adrenal cortex and what is produced in each layer?
- Zona glomerulosa* = Mineralocorticoids
- Zona fasciculata* = Glucocorticoids
- Zona reticularis* = Sex steroids (androgens and oestrogens)
GFR = Salt, sugar, sex
What is the (organic chemistry wise) difference between aldosterone and cortisol
Aldosteone lacks hydroxyl group on C-17
(Zona glomerulosa (i.e. aldosterone zone) doesnt have the enzyme 17α-hydroxylase)
Adrenal corticoids are synthesized from cholesterol. Enzymatic cleavage of a carbon side chain within mitochondria produces the C-21 steroid pregnenolone. Within cells of the zona fasciculata and zona reticularis, pregnenolone is hydroxylated at C-17 to form the glucocorticoid molecule. Zona glomerulosa cells lack 17α-hydroxylase; absence of a hydroxyl group on C-17 is the major difference between aldosterone and cortisol.
How is cortisol transported around body
And aldosterone
Steroid hormones are lipids so reliant onprotein-binding for transport
Cortisol:
- 75% bound to transcortin (=cortisol-binding globulin)
- 15% bound to albumin
- 10% unbound
Aldosterone:
- 10% bound to transcortin
- 50% bound to albumin
- 40% free
Where is transcortin produced?
Liver
Where are cortisol and aldosterone metabolised
Liver
What is half life of cortisol?
And aldosterone?
Cortisol half life 60 minutes
Aldosterone half life 20 mins
How is transcortin affected by pregnancy
Pregnancy increases hepatic synthesis of transcortin
(wonder why…)
What is the primary function of glucocorticoids?
List 3 other effects of glucocorticoids
Regulation of metabolism
(particluarly by stimulation of hepatic gluconeogenesis)
Other effects:
- Protein catabolism
- Protein sythesis inhibition
- Suppression of inflammatory/immune reponse
- Gastric acid secretion
- Vasopressin inhibition
- Lipolysis stimulation
- Inhibition of glucose uptake and metabolism
Describe the hormonal axis for glucocorticoids
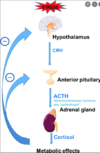
What are the major functions of aldosterone?
Electrolye balance
BP homeostasis
(–> retention of water, sodium and chloride, excertion of potassium)
Describe the cycle controlling aldosterone release.
- Release of aldosterone is influenced primarily by the RAAS and blood potassium concentrations.
- Renin, which is produced by the juxtaglomerular apparatus of the kidney, is a proteolytic enzyme that splits circulating angiotensinogeN (synthesized in the liver) into angiotensin I.
- (Within the pulmonary capillary endothelium) angiotensin I is converted to angiotensin II by angiotensin-converting enzyme (ACE).
- Angiotensin II stimulates peripheral vasoconstriction and secretion of aldosterone by the zona glomerulosa.
- Aldosterone in turn promotes sodium, chloride, and water reabsorption and potassium excretion, particularly at the renal tubules.

Where is renin produced?
Where is angiotensiongen produced?
Where is ACE produced?
Where is aldosterone produced?
Renin: from juxtaglomerular apparatus of kindey
Angiotensinogen: from liver
ACE: from pulmonary capillary endothelium
Aldosterone: from zona glomerulosa of adrenal gland

Where are cathecholamines produced?
Chromaffin cells of adrenal medulla
What is the substrate and enzyme that lead to cathecholamine production
Substrate: Tyrosine (and a little bit of phenylalanine)
Enzyme: Tyrosine hydroxylase
Product: Cathecholamines (dopa, dopamine, norepinephrine, epinephrine)
What system regulates adrenal medulla
Sympathetic nervous system
What is the ratio of epinephrine: norepinephrine in dogs?
And in cats?
Dogs:
60% epinephrine, 40% norepinephrine
Cats:
70% epinephrine, 30% norepinephrine
How are cathecholamines metabolised and extreted?
Metabolised by liver
Secreted by kidneys
WHat is the primary action of cathecholamines?
Acute stress response
Regulation of intermediary metabolism (particularly in response to hypoglycaemia)
Describe the action of epinephrine and norepi:
- Actions are mediated through alpha- and beta-adrenergic receptors on target tissues.
- Alpha-1 and alpha-2 receptors control catecholamine release from presynaptic and postsynaptic sympathetic nerve endings.
- Beta-1 receptors primarily affect the heart, and beta-2 receptors affect intermediary metabolism and smooth muscle contraction.
- Epinephrine is approximately 10 times more potent on beta-2 receptors (–> relaxation of smooth muscle in bronchioles, detrrusor muscle, GI, iris) than norepinephrine, so it is more important in controlling metabolism.
- At beta-2 receptors, epinephrine increases blood glucose concentrations, particularly by promoting hepatic glycogenolysis and gluconeogenesis. Epinephrine also stimulates glycogenolysis in skeletal muscle, with subsequent production of lactate that is converted by the liver to glucose.
- Epinephrine inhibits insulin secretion (via alpha-2 receptors), stimulates pancreatic glucagon secretion to increase blood glucose concentrations, and promotes lipolysis to increase free fatty acid concentrations in the blood.
- Epinephrine and norepinephrine interact with beta (1) receptors to increase the force of cardiac contraction and, by shortening the duration of diastolic depolarization, increase heart rate.
- Norepinephrine causes generalized vasoconstriction through stimulation of alpha receptors, resulting in an increase in vascular resistance and systolic blood pressure. In contrast, epinephrine’s affinity for beta-2 receptors causes vasodilation in skeletal muscle arterioles, coronary arteries, and all veins. Although this reduced peripheral resistance should decrease diastolic pressure, minimal change in blood pressure is usually noted because of the concurrent increase in cardiac output secondary to an increased heart rate and contractility produced by epinephrine.

List 3 features that can used to diagnose an adrenal ‘mass’
- >1.5cm maximum width
- Loss of typical kidney-bean shape
- Asymmetric shape and size compared with contrlateral gland
List 4 other ddx for an adrenal mass (based on asymmetry)
- Cyst
- Haematoma
- Normal hypertrophy
- inflammatory nodule




