Chapter 12- WBC: Neoplastic Proliferations of White Cells Flashcards
Malignancies are clinically the most important disorders of white cells. These diseases fall into
several broad categories:
- Lymphoid neoplasms
- Myeloid neoplasms
- histiocytoses
What are Lymphoid neoplasms?
Lymphoid neoplasms include a diverse group of tumors of B-cell, T-cell, and NK-cell
origin.
In many instances the phenotype of the neoplastic cell closely resembles that of
a particular stage of normal lymphocyte differentiation, a feature that is used in the
diagnosis and classification of these disorders
Where do Myeloid neoplasms arise?
early hematopoietic progenitors.
What are the three categories of
myeloid neoplasia that are recognized:
- acute myeloid leukemias, in which immature
- myelodysplastic syndromes
- chronic myeloproliferative disorders,
What are cute myeloid leukemias?
in which immature
progenitor cells accumulate in the bone marrow
What are myelodysplastic syndromes?
which are
associated with ineffective hematopoiesis and resultant peripheral blood cytopenias
What are chronic myeloproliferative disorders?
, in which increased production of one or more
terminally differentiated myeloid elements(e.g., granulocytes) usuallyleads to elevated
peripheral blood counts.
What are histiocytoses?
uncommon proliferative lesions of macrophages and dendritic cells.
Although “histiocyte” (literally, “tissue cell”) is an archaic morphologic term, it is still often used.
A special type of immature dendritic cell, the Langerhans cell, gives rise to a spectrum of neoplastic disorders referred to as the Langerhans cell histiocytoses
A special type of immature dendritic cell, the Langerhans cell, gives rise to a spectrum of neoplastic disorders referred to as the _________
Langerhans cell histiocytoses
ETIOLOGIC AND PATHOGENETIC FACTORS IN WHITE CELL NEOPLASIA: OVERVIEW
As we will see in the following sections, the neoplastic disorders of white cells are extremely
varied. Before we delve into this complexity, it is worth considering a few themes of general
relevance to their etiology and pathogenesis
- Chromosomal Translocations and Other Acquired Mutations
- Inherited Genetic Factors.
- Viruses.
- Chronic Immune Stimulation.
- Iatrogenic Factors
- Smoking.
Chromosomal Translocations and Other Acquired Mutations
What are present in the
majority of white cell neoplasms.?
As was discussed briefly in Chapter 7 , many specific
rearrangements are associated with particular neoplasms, suggesting a critical role in their
genesis.
Nonrandom chromosomal abnormalities, most commonly translocations
Chromosomal Translocations and Other Acquired Mutations
- The genes that are mutated or otherwise altered often play crucial roles in the development, growth, or survival of the normal counterpart of the malignant cell
- Oncoproteins created by genomic aberrations often block normal maturation
- Proto-oncogenes are often activated in lymphoid cells by errors that occur during antigen receptor gene rearrangement and diversification
What are the result of the genes that are mutated or otherwise altered often play crucial roles in the
development, growth, or survival of the normal counterpart of the malignant cell?
- “dominant- negative” protein interferes with a normal function (a loss of function
- inappropriate increase in some normal activity (a gain of function)
- certain tumors, different aberrations have the same functional consequence as a result of their convergence on a common critical signaling pathway or transcription factor
*
Give an example of different aberrations have the same functional consequence as a result of their
convergence on a common critical signaling pathway or transcription factor.
“MALTomas”
B-cell lymphomas
occurring in extranodal mucosal sites, which are often associated with translocations
involving either the MALT1 or the BCL10 gene.
The MALT1 and BCL10 proteins bind
one another in a protein complex that regulates NF-κB, a transcription factor with important pro-survival functions in normal lymphocytes.
The net effect of translocations
involving either MALT1 and BCL10 is the same—a dysregulation of the MALT1/BCL10 complex that causes the constitutive activation of NF-κB, [5] which (as we will see) plays a role in the pathogenesis of many lymphoid malignancies
Many oncoproteins cause an arrest in differentiation, often at a stage when cells are proliferating rapidly. The importance of this block in maturation is most evident in the what?
acute leukemias, in which dominant-negative mutations involving transcription factors that interfere with early stages of lymphoid or myeloid cell differentiation often
collaborate with activating mutations in tyrosine kinases that increase cell survival and
proliferation ( Fig. 13-4 ).
However, this theme also applies to more mature lymphoid tumors. For example, BCL6 encodes a transcription factor that is expressed in germinal
center B cells. Without BCL6, germinal center B cells cannot form; however, BCL6 must
be turned off for germinal cell B cells to mature into memory B cells or plasma cells. As
we will discuss, aberrations that up-regulate BCL6 expression and prevent its downregulation
are very common in certain types of lymphomas derived from germinal center
B cells.
Proto-oncogenes are often activated in lymphoid cells by what?
errors that occur during antigen receptor gene rearrangement and diversification.
Among lymphoid cells, potentially oncogenic mutations occur most frequently in where?
germinal center B cells during
attempted antibody diversification.
After antigen stimulation, B cells enter germinal
centers and upregulate the expression of activation-induced cytosine deaminase (AID),
a specialized DNA-modifying enzyme that is essential for two types of Ig gene modifications: class switching, an intragenic recombination event in which the IgM
heavy-chain constant gene segment is replaced with a different constant segment (e.g., IgG3), thus allowing an isotype switch of antibody class; and somatic hypermutation, which creates point mutations within Ig genes that may by chance increase antibody
affinity for antigen ( Chapter 6 ).
Give a certain example wherein a proto-oncogenes are activated in germinal center B- cell lymphomas by chromosomal translocations involving class switch regions?
Certain proto-oncogenes, such as c-MYC, are
activated in germinal center B-cell lymphomas by chromosomal translocations involving
class switch regions.
Remarkably, AID expression is sufficient to induce c-MYC/Ig translocations in normal germinal center B cells, [8,] [9] apparently because it creates
lesions in DNA that lead to chromosomal breaks
Parenthetically, it follows that even
activation of a strong oncogene like c-MYC is not sufficient to cause transformation,
emphasizing that (like other cancers) lymphomas arise due to a combination of multiple
genetic lesions. Other
What Other proto-oncogenes,are more commonly activated in
germinal center B-cell lymphomasbypoint mutations,[10] which also seem tostem from
“mistargeting” of AID.
BCL6
Undoubtedly, the selective advantage offered by antibody diversification against infection far outweighs the price that is paid in terms of potentially
oncogenic mutations, but this is of little solace to individuals afflicted with tumors of
germinal center B-cell origin, which include the most common and clinically important
lymphoid neoplasms.
What is V(D)J recombinase?
A different type of regulated genomic instability is unique to precursor B and T cells, which express a V(D)J recombinase that cuts DNA at specific
sites within the immunoglobulin (Ig) and T-cell receptor loci, respectively.
This process is
essential for the assembly of productive antigen receptor genes, but sometimes goes
awry, leading to the joining of portions of other genes to antigen receptor gene
regulatory elements.
Particularly in tumors of precursor T cells, proto-oncogenes are often deregulated by their involvement in such aberrant recombination events.
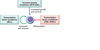
FIGURE 13-4 Molecular pathogenesis of acute leukemia. Acute leukemias arise from
complementary mutations that block differentiation at early stages of white cell development,
enhance self-renewal, and increase growth and survival. Important examples of each type of
mutation are listed. BCR-ABL, breakpoint chromosomal region–Abelson kinase fusion gene;
MLL, mixed-lineage leukemia gene; PML-RARα, promyelocytic leukemia–retinoic acid
receptor α fusion gene
What are genetic diseases that promote genomic instability are at increased risk of acute leukemia?
- Bloom syndrome,
- Fanconi anemia, and
- ataxia telangiectasia
What Inherited Genetic Factors are associated with an increased incidence of childhood leukemia?
- Down syndrome (trisomy 21) and
- type I neurofibromatosis
What are the three lymphotropic viruses
- viruses—human T-cell leukemia virus-1 (HTLV-1),
- Epstein-Barr virus (EBV),
- Kaposi sarcoma herpesvirus/human herpesvirus-8 (KSHV/HHV-8)


