Carcinogens, Cancer Epi, and Clinical Manifestations Flashcards
What are the high-risk HPV types? Why?
- HPV 16, 18, 31
- Implicated in the pathogenesis of squamous cell carcinomas of the cervix, anogenital region, and head and neck (particularly tumors arising in the tonsillar mucosa)
- These cancers are sexually transmitted infections, caused by transmission of HPV
What % of women with breast cancer have no symptoms of it at time of dx and it is detected by screening with mammography?
55%
Describe how hepatocellular injury via chronic viral infection may lead to increased carcinogenesis.
- Leads to compensatory proliferation of hepatocytes
- Regenerative process aided by growth factors, cytokines, chemokines, and o/bioactive substances produced by activated immune cells -> promote cell survival, tissue remodeling, and angiogenesis
- Activated immune cells also produce other mediators, like ROS, that are genotoxic and mutagenic.
- 1 key molecular step is activation of NF-κB pathway in hepatocytes in response to mediators derived from the activated immune cells -> activation of this pathway in hepatocytes blocks apoptosis, allowing dividing cells to incur genotoxic stress and accumulate mutations
How has melanoma genome sequencing been used to reinforce the belief that sun exposure has an important causative role in this disease?

- Has revealed a very large number of mutations that appear to stem from non-templated, error-prone repair of pyrimidine dimers, reinforcing the belief that sun exposure has an important causative role in melanoma
What are nitrites?
- Used as food preservatives, and cause nitrosylation of amines contained in the food
- The nitrosoamines so formed are suspected to be carcinogenic
Once injected into a gastric mucosal epithelial cell, what does the virulence factor, CagA do?

- CagA PAI also encodes a type 4 secretion sys used to “inject” CagA into target cell upon H. pylori attachment
- CagA localizes to inner surface of cell membrane and undergoes tyrosine phosphorylation via Src family kinases -> phosphorylated CagA interacts with SHP-2 tyrosine phosphatase, so functionally active, triggering a host cell morphological change to more motile pheno
- This phenotype mimics effect produced by hepatocyte growth factor which may participate in various aspects of cancer, including metastasis (+ RAS, + MEK -> image)
- CagA a highly Ag protein associated with a prominent inflammatory response by eliciting IL-8 production
- Both CagA + and - strains; around 60% in Western countries positive, but majority in East Asian positive
How do translocations activate proto-oncogenes?
- While any type of chrom rearrangement can activate proto-oncogenes, translocation is most common mech
- Can activate proto-oncogenes in two ways:
1. Promoter/enhancer substitution: overexpression of proto-oncogene by swapping its regulatory elements with those of another gene, esp. one that is highly expressed
2. Formation of fusion gene: coding sequences of 2 genes fused in part or whole -> expression of novel chimeric protein w/oncogenic properties
What is going on in this stomach sample?

Cancer cells invading (top right and lower left)
What are the 3 acquired conditions that predispose to cancer?
- Chronic inflammatory disorders
- Precursor lesions
- Immunodeficiency states
How is epigenetics related to carcinogenesis?
- Epigenetics: factors other than the DNA seq that regulate gene expression -> important role in aspects of malignant phenotype, incl expression of cancer genes, control of differentiation and self-renewal, and even drug sensitivity and drug resistance
- Factors include:
1. Histone modifications catalyzed by enzymes associated with chromatin regulatory complexes
2. DNA methylation via DNA methyltransferases
3. Other proteins that regulate higher order org of DNA (e.g., looping enhancer elements on gene promoters)
How can proto-oncogenes and TSGs be tumor antigens?
- Products of altered proto-oncogenes, TSGs, and “passenger” genes translated in cyto of tumor cells and entering MHC I where they may be recognized via CD8
- May also enter MHC II in APCs that phagocytose dead tumor cells, and be recognized by CD4+ T cells, too
- Some pts have circulating CD4+ and CD8+ T cells that can respond to peptides from mutated oncoproteins like RAS, p53, and BCR-ABL.
How is HPV genome integration implicated in malignant transformation?
- While site of viral integration in host chromosomes is random, pattern of integration is clonal -> cells in which viral genome has integrated show significantly more genomic instability
- B/c integration site random, no consistent assoc with a host proto-oncogene, but, integration interrupts viral DNA in E1/E2 open reading frame, so loss of E2 viral repressor & overexpression of oncoproteins E6 and E7
- Oncogenic potential of HPV can largely be explained byactivities of the two viral genes encoding E6 and E7
What causes the carcinogenicity of UVB light?
- Due to formation of pyrimidine dimers in DNA
- If the energy in a photon of UV light is absorbed by DNA, a chemical rxn occurs, leading to covalent cross-linking of pyrimidine bases, esp. adjacent thymidine residues in the same strand of DNA
- Distorts DNA helix and prevents proper pairing of dimer with bases in opposite DNA strand
- Pyrimidine dimers are repaired via nucleotide excision repair pathway, but this pathway can be overwhelmed w/excessive sun exposure
Pause. And reflect. Also, memorize this graphic.

Good job!
What are these? How do they relate to neoplasia?

- Cafe au lait spots
- Neurofibromatosis Type 1 (NF1): auto dom disorder caused by mutations in TSG, neurofibromin, a negative regulator of the oncoprotein, Ras
- Disruption of neurofibromin function, leading to Ras hyperactivity; cardinal feature of NF1-associated tumors
- As would be anticipated for a tumor suppressor gene, sole normal neurofibromin allele mutated or silenced in tumors arising in NF1, which include neurofibromas
- Symptoms: learning disabilities, seizures, skeletal abnormalities, vascular abnormalities, pigmented iris nodules (Lisch nodules), and pigmented skin lesions (axillary freckling, café au lait spots) in various degrees
What is this? Describe the symptoms, mechanisms and associated neoplasias.

- Coalescing nests of intestinal carcinoid (carcinoid syndrome)
- Symptoms: attacks of cutaneous flushing (deep red erythema of face and neck; may become persistent erythema or cyanosis), diarrhea, cramps, nausea, cough, vomiting, etc.
- Mechanisms: serotonin, bradykinin (vasodilator)
- Neoplasias: bronchial adenoma (carcinoid), pancreatic, gastric carcinoma
What is the difference between lymphomas and leukemias?
- Lymphomas: solid hematologic malignancies
- Leukemias: liquid hematologic malignancies
How do HPV proteins promote many of the hallmarks of cancer?
- High-risk HPV types express oncogenic proteins that:
1. Inactivate tumor suppressors (p53, Rb, p21, p27)
2. Activate cyclins (E and A)
3. Inhibit apoptosis, and
4. Combat cellular senescence - NOTE: the primacy of HPV infection in the causation of cervical cancer is confirmed by the effectiveness of HPV vaccines in preventing cervical cancer
Diagnose this Pap.

LSIL (low-grade squamous epithelial lesion)
What is this?

Intestinal metaplasia due to H. pylori -> precursor to dysplasia and carcinoma
What is this? What is a potential precursor?
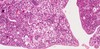
- Endometrial adenocarcinoma, potentially predicated by simple endometrial hyperplasia
What are some examples of the carcrinogenicity of radiation?
- Electromagnetic (x-rays, γ rays) and particulate (α particles, β particles, protons, neutrons) radiations
- Miners of radioactive elements in Rocky Mountains have 10x increased incidence of lung cancers
- Follow-up of survivors of atomic bombs: initially marked increase in incidence of certain leukemias after avg latent period of about 7 years; later, incidence of many solid tumors with longer latent periods (e.g., carcinomas of the breast, colon, thyroid, and lung) increased
I know this says what it is. Just observe.

Cervical squamous cell carcinoma
Describe the actions of the HPV E7 virulence factor.
- E7 protein effects complement those of E6, centered on moving cells past G1/S cell cycle checkpoint
- Binds **Rb **protein, displacing E2F transcription factors normally sequestered by Rb, promoting progression through cell cycle (high-risk HPV E7 has higher affinity for Rb than low-risk HPV)
- E7 also inactivates CDK inhibitors p21 and p27
- High-risk HPVs (types 16, 18, and 31) E7 also binds and presumably activate cyclins E and A
Describe why CT scans are a concern for child health.
- Widespread use of computerized tomography (CT scans)
- Studies show that children who get 2 or 3 CT scans have 3x higher risk of leukemia, and those that have 5 to 10 scans have a 3x higher risk of brain tumors
- Overall risk in children is very low (roughly one excess leukemia and one excess brain tumor over 10 years per 10,000 CT scans), but emphasizes the need to minimize radiation exposure whenever possible
What is the difference b/t HPV genome integration in benign vs. cancerous papillomas? Why is this important?
- Benign warts: HPV genome maintained in non-integrated episomal form
- Cancers: HPV genome integrated into host genome
- Suggests that integration of viral DNA important for malignant transformation
How does DNA amplification lead to overexpression of oncogenes? Describe the two most important amplifications.
- May produce up to several hundred copies of the oncogene in the tumor cell
- Two mutually exclusive patterns are seen:
1. Multiple small extrachromosomal structures called double minutes
2. Homogeneous staining regions (HSRs): inserts of amplified genes in new chrom locations that may be distant from normal oncogene location - 2 most important amplifications are:
1. NMYC: amplified in 25%-30% of neuroblastomas, and associated with poor prognosis
2. ERBB2: AMP in 20% of breast cancers -> Ab therapy directed against HER2 receptor encoded by ERBB2 an effective therapy (Trastuzumab)
Describe the epidemiology of childhood cancer.
- Accounts for 10% of deaths in children under 15 in US; about 60% of these deaths due to acute leukemias and brain tumors
- Very different types than those that occur in adults: leukemia, neuroblastoma, Wilms, retinoblastoma, and rhabdomyosarcoma most common in children (and carcinomas extraordinarily rare)
- Children get brain tumors more than adults, inlc. types that don’t occur in adults; most are technically benign, but tend to be fatal b/c can’t be sx removed, and often don’t respond to non-sx therapies, so their autonomous growth in un-expandable skull compresses vital brain structures
How do chromosomal deletions cause loss of TSGs or the formation of oncogenes. Provide some examples.
- Very prevalent structural abnormality in tumor cells
- Deletion of specific regions of chroms associated with the loss of particular TSGs:
1. Deletions involving chromosome 13q14, site of the Rb gene, associated with retinoblastoma
2. Deletion of the VHL on chromosome 3p a very common event in renal cell carcinomas - Not all deletions lead to loss of gene function; a few activate oncogenes through same mechanisms as chromosomal translocations:
1. Deletions on chrom 2q in subset of lung cancers produce an oncogenic EML4-ALK fusion gene encoding a constitutively active tyrosine kinase
2. It is likely that more “cryptic” deletions that activate oncogenes will be discovered
Why is there geographic variation in cancer prevalence?
- Thought to mainly stem from different environmental exposures
- Important environmental factors implicated in carcinogenesis include: infectious agents, smoking, alcohol, diet, obesity, reproductive history, and carcinogens
What is the diffuse disease? What about the concentrated pathologic area on the bottom right?

- Liver cirrhosis from Hep C
- Hepatocellular carcinoma
What are these two images showing?

How an immunostain can reveal tumor cells (this is a lymph node)
What are these?

Acute leukemia cells in bone marrow aspirate
What are the major risk factors for colon cancer?
- Hereditary forms of colorectal cancer
- Personal or family history of sporadic colorectal cancer (and possibly large or advanced adenomas)
- Age
- Inflammatory bowel disease (IBS)
- Several potentially modifiable risk factors: obesity, lack of physical activity
What is this?

- Normal, baseline uterine cervical squamous epithelium
- The basal cell layer looks a little thicker than a single cell, but this is still normal
- Clear cytoplasm due to glycogen storage and flattening of cells are signs of maturation in cervical squamous epithelium
How are chronic inflammation and precursor lesions carcinogenic?
- Diverse set of conditions all associated with increased cellular replication, which appears to create fertile soil for devo of malignant tumors with repeated round of cell division, accumulating genetic lesions leading to carcinogenesis
1. Tumor arising in chronic inflammation mostly carcinomas, but also include mesothelioma and several types of lymphoma
2. Virtually all precursor lesions arise in epithelial surfaces and are associated with an increased risk of carcinoma
Name 3 malignancies with a higher incidence in immunodeficient patients, and their associated viruses.
- Pts with immunodeficiency have a higher incidence of malignancies of certain types, incl:
1. B-cell lymphomas of B-cells infected with Epstein-Barr virus (EBV).
2. Kaposi sarcoma: a proliferation of endo cells due to human herpes virus 8 (HHV 8).
3. Squamous cell carcinomas of cells infected with oncogenic strains of human papilloma virus (HPV)
What is H. pylori?
- Gram (-) microaerophilic, spiral-shaped bacterium with unipolar flagellae uniquely adapted to living in human stomach
- The primary etiologic agent of gastric cancer
Exposure to UV rays from the sun is associated with an increased incidence of which cancers?
- Squamous cell carcinoma
- Basal cell carcinoma
- Skin melanoma
Immunodeficiency states predispose to what types of cancers?
Virus-induced (i.e., HPV)
Describe the epidemiology of hepatocellular carcinoma in Western countries.
- Incidence rapidly increasing, largely due to HCV epidemic -> tripled in the US in recent decades
- In Western populations, hepatocellular carcinoma rarely manifests before age 60, and in almost 90% of cases, tumors develop in the setting of cirrhosis
- There is a pronounced male predominance, about 3:1
What are paraneoplastic syndromes? List 3 important characteristics.
- Symptoms not attributable to direct effects of tumor (or hormones native to primary tumor organ)
1. Can be earliest manifestation of occult tumor
2. Can be sickening, even fatal by themselves
3. May mimic metastatic disease - Occur in about 10% of cancer patients
- NOTE: cachexia is in a class by itself and does not count as a paraneoplastic syndrome
What is the dominant mechanism in the pathogenesis of virus-induced hepatocellular carcinoma?
Activation of the NF-κB pathway
Briefly describe promotion (i.e., in carcinogenesis).
- Promoters can induce tumors to arise from initiated cells, but are nontumorigenic by themselves
- Cellular changes resulting from the application of promoters do not affect DNA directly and are reversible
- Enhance the proliferation of initiated cells
What gene in the HBV genome may directly promote the development of cancer? What other characteristic of HBV contributes to its carcinogenicity?
- HBx: can activate a variety of transcription factors and signal transduction pathways
- In addition, viral integration (DNA virus) can cause structural changes in chromosomes that dysregulate oncogenes and tumor suppressor genes (TSG)
What is up with this oral mucosa?

- Leukoplakia with severe dysplasia -> basaloid cells failing to mature and flatten out, extending almost all the way to the top
Describe the actions of the HPV E6 virulence factor.
- E6 protein binds to and mediates degradation of p53
- Stimulates expression of TERT, the catalytic subunit of telomerase that contributes to immortalization of cells
- High-risk HPV E6 has higher affinity for p53 than E6 from low-risk HPV types
- Human TP53 is polymorphic at codon 72, encoding a proline or arginine residue at that position -> p53 Arg72 variant much more susceptible to degradation by E6 and infected individuals with the Arg72 polymorphism are more likely to develop cervical carcinomas
What does this patient have? Name the symptoms, potential mechanisms, treatments, and associated forms of neoplasia.

- Hypercalcemia of malignancy
- Symptoms: nausea, vomiting, constipation, polyuria, disorientation, lethargy, seizures
- Mechanism: parathyroid hormone- related protein (PTHRP), TGF-alpha (polypeptide factor that activates osteoclasts), TNF, IL-1
- Treatments: hydration, bisphosphonates
- Neoplasias: squamous cell carcinoma of lung (most common cause), breast, renal, ovarian carcinoma, or adult T cell leukemia/lymphoma
What are tumor stage and tumor grade?
- **Tumor stage: **anatomic extent of the tumor, including primary tumor size, extent of lymph node and distant metastases
- Tumor grade: qualitative assessment of differentiation of tumor (extent to which it resembles normal tissue at the primary site)
Diagnose this.

LSIL (low-grade squamous intraepithelial lesion)
Is H. pylori infection treatable to prevent gastric carcinomas it causes?
Yes (complicated and evolving tx)
What is CIN? What are its grades? How are these related to SIL?
-
CIN = cervical intraepithelial neoplasia
1. CIN 1: squamous intraepithelial lesion (SIL)
2. CIN 2 and 3: high-grade SIL (unfortunately, low-grade, CIN 1, LSIL often looks the same as HSIL)
This is gastric surface mucosa. The arrowhead points to normal. The arrow points to a cell type NOT normally present in the gastric mucosa; what is it? What condition does this represent? Why is it important?

- Goblet cell
- Intestinal metaplasia
- Risk of developing gastric carcinoma
What is this a sign of? What are the other signs and symptoms, mechanisms, and associated neoplasias?

- CUSHING SYNDROME
- Signs/symptoms: weight gain, central obesity, moon face (fat deposition), weakness, hirsutism, depression, psychosis, hypertension, glu intolerance, blood red abdominal striae, buffalo hump dorsal neck fat deposition, menstrual irregularity, muscle wasting, etc.
- Mechanisms: ACTH or ACTH-like substance
- Neoplasias: pituitary adenoma (most common), small cell carcinoma of lung (2nd most common), pancreatic carcinoma, neural tumors
What is an indirect acting carcinogen?
Require metabolic conversion to become active “ultimate carcinogens”
What is this?

Keratin pearl
What do you think is going on here?

- Classic microscopic pathology of the most common type of melanoma
- Single cells, or small clusters of large round cells, with moderate amount of lightly basophilic cytoplasm, often with granules of brown melanin pigment and large nuclei with prominent nucleoli
How is prostate cancer usually dx? Do pts typically have symptoms at time of dx?
- Dx usually after screening with prostate specific antigen (PSA) or rectal exam
- Vast majority of prostate cancer pts. have no symptoms at time of dx
Degree of cancer risk from UV exposure depends on what factors?
- 1) Type of UV rays, 2) intensity of exposure, 3) quantity of light-absorbing “protective mantle” of melanin in skin
- Fair-skinned people with repeated sunburns have high skin cancer incidence
- Non-melanoma skin cancers associated with total cumulative exposure to UV radiation
- Melanomas associated with intense, intermittent exposure, as occurs with sunbathing
What bug is implicated in the pathogenesis shown here?

H. pylori (gastric adenocarcinoma)
What are some factors secreted by tumors to inhibit the host immune response?
- TGF-β: secreted in large quantities by many tumors and is a potent immunosuppressant
- Other tumors secrete: galectins (sugar-rich lectin-like factors that skew T-cell responses to favor immuno-suppression)
- Many other soluble factors produced by tumors are also suspected of inhibiting the host immune response, including: IL-10, PGE2, some metabolites of tryptophan, and VEGF, which can inhibit diapedesis of T cells from the vasculature into the tumor bed.
What is H. pylori, and how is it related to cancer?
- Common bacterial infection that causes chronic active gastritis -> prevalence increases with age
- More severe in atrum, with lymphocytic (and germinal center), neutrophil (making it active), and spiral bacteria in superficical mucus layer
- H. pylori cytotoxin-associated gene A (CagA) protein: 1) messes up cell polarization, 2) activates pathways to cell proliferation (via RAS, MEK, ERK, NF-kappaB, beta-catenin), and 3) causes degradation of p53
-
Precursor to gastric adenocarcinoma via intestinal metaplasia (similar to Barrett esophagus)
1. 1-3% leads to gastric adenocarcinoma, and 0.1% leads to low-grade lymphoma
Describe the nucleotide excision repair pathway, and why it is important for DNA repair (and cancer prevention).

- Pyrimidine dimers are repaired by nucleotide excision repair pathway -> five steps, > 30 proteins involved
- In excessive sun exposure, capacity of this pathway is overwhelmed, and error-prone non-templated DNA-repair mechanisms become operative, providing for the survival of the cell at the cost of genomic mutations, which, in some instances, lead to cancer
- Importance of the nucleotide excision repair pathway most graphically illustrated by high freq of cancers in people w/hereditary disorder xeroderma pigmentosum, a disorder characterized by defective nucleotide excision repair
What is going on here? I know, it’s too easy.

Cervical squamous cell carcinoma
What are direct-acting carcinogens?
- Require no metabolic conversion to be carcinogenic
- Most weak carcinogens, but some are chemotherapeutic drugs (e.g., alkylating agents) for leukemia, lymphoma and ovarian carcinoma -> these can later cause a second cancer, usually acute myeloid leukemia (AML)
What is the epidemiology of colon cancer (who gets it, symptoms, prognosis)?
- Typically patients over 60 y/o; 10 years older than avg adenoma patient
- Symptoms: abdominal pain (44%), change in bowel habits, i.e., diarrhea or constipation (43%), hematochezia (freshly bloody anus) or melena (black, tarry feces) (40%)
- Many colon cancer patients asymptomatic at time of dx screening colonoscopy -> 80% dx before metastatic
- Prognosis depends on stage: from 74% 5-year survival for stage I to 6% for stage IV
How are most chemical carcinogens metabolized?
- Most chemical carcinogens need metabolic activation for conversion into ultimate carcinogens -> some metabolic pathways may inactivate (detoxify) them
- Most known carcinogens metabolized by CYP-450-dependent mono-oxygenases
- Genes that encode these enzymes are polymorphic, and their activity and inducibility vary significantly in pop -> susceptibility to carcinogenesis related in part to particular polymorphic variants an individual inherits
How are Hep B and Hep C associated with cancer?
- Epi studies suggest close association b/t HBV and HCV and liver cancer -> up to 85% of hepatocellular carcinomas worldwide caused by infection w/HBV/HCV
- HBV endemic in Far East and Africa, so these areas have highest incidence of hepatocellular carcinoma
- Mode of action of these viruses in liver tumorigenesis not fully understood -> HBV and HCV genomes do not encode any viral oncoproteins, and although the HBV DNA is integrated within the human genome, there is no consistent pattern of integration in liver cells.
- Dominant effect may be immunologically mediated chronic inflammation and hepatocyte death leading to regeneration and genomic damage
- Also suspected that in setting of unresolved chronic inflammation, i.e., viral hepatitis or chronic gastritis via H. pylori, immune response may become maladaptive, promoting rather than preventing tumorigenesis
How is hypermethylation implicated in carcinogenesis? Provide an example.
- Some cancer cells exhibit selective hypermethylation of promoters of TSGs -> transcriptional silencing
- Hypermethylation usually occurs on only one allele and function of o/copy of affected TSG is lost through another mechanism, like disabling point mut or deletion
- Ex of TSG hypermethylated in several cancers CDKN2A, a complex locus that encodes two tumor suppressors, p14/ARF and p16/INK4a, that enhance p53 and RB activity, respectively
Name two carcinogenic viruses, and two oncofetal antigens that can be tumor antigens.
- HPV and EBV: recognized by CTLs -> competent immune system plays a role in surveillance against virus-induced tumors due to its ability to recognize and kill virus-infected cells
- Oncofetal antigens expressed at high levels on cancer cells can serve as markers that aid in tumor diagnosis and clinical management -> two most thoroughly characterized are carcinoembryonic antigen (CEA) and alpha-fetoprotein (AFP)
What is going on in this sample of hepatocytes?

- Larger cells with bigger nuclei, higher ratio of nucleus to cytoplasm, pleomorphism, some abnormally shaped nuclei or abnormally clumped chromatin in the middle of the field
- This is dysplasia, not anaplasia, although it has similar features (anaplastic cells would exhibit much more irregularity because this is an irreversible condition)
What is going on here?

- Ciliated pseudostratified columnar respiratory epi falling off very thick basement membrane overlying an infiltrate of small cells with inconspicuous cytoplasm and nuclei a bit too big and irregular to be lymphocytes
- Small cell carcinoma: highly malignant cancer that commonly arises in lung (but can also be in cervix, prostate, or GI)
- Perhaps the type of cancer most likely to cause a para- neoplastic syndrome because a neuroendocrine tumor:
1. Neoplasms that arise from cells of the endocrine (hormonal) and nervous systems. Many are benign, but some are malignant; most commonly occur in the intestine, where often called carcinoid tumors, but also found in pancreas, lung and rest of body
Diagnose this Pap smear.

Normal
What are the three wavelength ranges of the UV portion of the solar spectrum? Which of these is most responsible for the induction of cutaneous cancers?
- UVA (320-400 nm), UVB (280-320 nm), and UVC (200-280 nm)
- UVB believed to be responsible for the induction of cutaneous cancers
What is a precursor lesion?
- Localized morphologic changes associated with a high risk of cancer
- Virtually all precursor lesions arise in epithelial surfaces and are associated with an increased risk of carcinoma
What is this? What is it an example of?

- Endometrial hyperplasia
- One of the most common non-inflammatory hyperplasia precursor lesions -> caused by sustained estrogenic stimulation of endometrium
What is going on here in A, B, and C? How can you tell which examples are “worse?”

A. Mild
B. Moderate
C. Severe
- Dysplasia causes cells higher in the epithelium to look more basal, i.e., less flattened, with less glycogen -> the higher in the epithelium these cells are, the worse the dysplasia
Does everyone infected with H. pylori get gastric cancer?
No - only about 2-3% of infected individuals
How do tumor cells inhibit tumor immunity by engaging normal “checkpoints?”
- May down-regulate expression of costimulatory factors on APCs, so fail to engage CD28 and instead activate inhibitory receptor CTLA-4 on effector T cells -> this not only prevents sensitization, but also may induce long-lived unresponsiveness in tumor-specific T cells
- Tumor cells also may upregulate expression of PD-L1 and PD-L2, cell surface proteins that activate programmed death-1 (PD-1) receptor on effector T cells. PD-1, like CTLA-4, may inhibit T cell activation
- Abs that block inhibitory CTLA-4 or PD-1 receptors have produced promising results in clinical trials in patients with advanced-stage solid tumors
- Success of these agents has led to a new paradigm in cancer immunotherapy, sometimes called “checkpoint blockade” -> idea that treatments that remove “brakes” imposed by tumors on host anti-tumor immune responses can be highly effective in treating cancer
In what age group are cancers most common?
Adults older than 60
What is this?

Normal intestinal epithelium
What is leukoplakia? What about erythroplakia?

- A thickening of squamous epithelium that may occur in oral cavity, or on the penis or vulva, and give rise to squamous carcinoma (relatively freq precursor lesion)
- Leukoplakia of the oral cavity associated w/smoking, and generally reversible -> patch of white thickening, often with hyperkeratosis
- Erythroplakia: related lesion, which tends to not be hyperkeratotic, but is often already carcinoma in situ, so is beyond precursor
What is the second step in the dx of cancer? Explain your answer.
-
Specific Dx: biopsy (most common, and usually best method)
1. Fine needle aspiration cytology: dx procedure to investigate superficial lumps/masses (thin hollow needle for collection, followed by staining)
2. Exfoliative cytology: i.e., Pap smear - +/- immunochemistry, flow cytometry, and molecular testing
What are the 4 most common symptoms of lung cancer? What % of ptshave symptoms at time of dx?
- Cough: up to 75%
- Hemoptysis: up to 50%
- Dyspnea: 25%
- Chest pain (20%) and weight loss
- 90% of lung cancer pts have symptoms at time of dx
What is CYP1A1?

- P450 gene responsible for the metabolism of polycyclic aromatic hydrocarbons, i.e., benzo[a]pyrene (cigs)
- About 10% of white pop has highly inducible form of this enzyme associated with increased risk of lung cancer in smokers
- Light smokers with susceptible CYP1A1 genotype have a 7x higher risk of developing lung cancer compared with smokers without the permissive genotype
- NOTE: attached image is a (+) immunostain for CYP1A1
What is this?

- Normal, baseline uterine cervical squamous epithelium
- Note the single layer of basal cells, and how the higher cells get flatter, with flatter nuclei
- Also not the abundant glycogen in the cytoplasm
What % of women are with breast cancer are dx with mammographically occult cancer?
15%
What is this immunostain showing?

Cancer cells infiltrating walls of stomach (of course, you can’t tell this is stomach)
What is shown here?

Endometrial hyperplasia caused by sustained estrogenic stimulation
Describe what is meant by lineage restricted, and its relationship to cancer epigenetics.
- Has an epigenetic basis -> TSGs and oncoproteins can be broadly divided into two classes:
1. Those mutated or otherwise dysregulated in many cancers (e.g., RAS, MYC, p53), and
2. Those mutated in a restricted subset of tumors (e.g., VHL in renal cell carcinomas, APC in colon carcinoma) and are thus lineage restricted - Cancer cell’s lineage/differentiation state generated by epigenetic modifications that produce a pattern of gene expression that characterizes that particular cell type
- Lineage-restricted cancer genes only act in epigenetic contexts in which key oncogenic targets are controlled by these genes
Describe the differential evolution of colon adenomas and carcinomas. Use the attached figure, if needed. Why is this important?

- Most colon carcinomas evolve through a series of morphologically identifiable stages: 1) colon epithelial hyperplasia, 2) adenomas that progressively enlarge, and 3) ultimately undergo malignant transformation
- Molecular analyses of proliferations at e/stage shows precancerous lesions have fewer mutations than adenocarcinomas, suggesting tendency to acquire particular mutations:
1) Inactivation of the APC TSG, 2) Activation of RAS, 3) Loss of TSG, SMAD, on 18q, 4) Loss of TP53 - Precise temporal sequence of mutations, incl gain of oncogenes and loss of TSGs needed for carcinogenesis varies by organ and tumor type, and individual tumor
How does chronic inflammation predispose to cancer?
- Tissue injury w/compensatory proliferation of cells to repair the damage -> also may increase pool of tissue stem cells, which may be particularly susceptible to transformation
- Activated immunce cells produce ROS (directly genotoxic) and inflammatory mediators that may promote bystander cell survival, even in face of genomic damage
- Chronic epi injury may lead to metaplasia (change to cell type better able to survive ongoing insult). Short term changes can be adaptive, but over longer time (years to decades), changes may allow cells with potentially oncogenic mutations to survive, eventually leading to cancer
What are polycyclic hydrocarbons?
- Some of most potent indirect chemical carcinogens
- Present in fossil fuels
- Benzo[a]pyrene (polycyclic aromatic hydroC found in coal tar) formed during high-temp tobacco combustion in cigs and implicated in causation of lung cancer
- May also be produced from animal fats via broiling or grilling meats, and present in smoked meats and fish
- Principal active products in many hydroCs epoxides, which form covalent adducts (addition products) with molecules in cell, mainly DNA, but also RNA & proteins
Briefly describe initiation (i.e., in carcinogenesis).
- Occurs in a cell from exposure to a carcinogenic agent sufficient to make it potentially capable of giving rise to a tumor
- Necessary, but not sufficient for tumor formation; must be combined with promotion to result in a neoplasm
- Causes permanent DNA damage (mutations); it is rapid, irreversible and has “memory”
- Tumors can result even if the promotion is delayed
What is aflatoxin B? Describe its pathogenic effect.
- Example of a chemical carcinogen that interacts preferentially with particular DNA sequences/bases, producing mutations clustered at “hotspots” enriched for particular base substitutions
- Produced by some strains of Aspergillus, which grows on grains and nuts -> strong correlation b/t dietary level of this food contaminant and incidence of hepatocellular carcinoma in parts of Africa and the Far East
- Aflatoxin B1-associated hepatocellular carcinomas tend to have particular mutation in TP53, a G:C→ T:A transversion in codon 249 that produces an arginine to serine substitution in the p53 protein -> TP53 mutations infrequent in liver tumors from areas where aflatoxin contamination of food doesn’t occur, and few of these mutations involve codon 249
Is infection with HPV sufficient for carcinogenesis? Explain the implications of this virus’ carcinogenicity.
- No: HPV acts in concert w/genetic & environmental factors, incl. cig smoking, coexisting microbial infection, diet deficiency, & hormonal changes, to cause cancer
- High proportion of women infected w/HPV clear the infection by immunologic mechanisms; others don’t, some for unknown reasons, some because of acquired immune abnormalities, such as those from HIV infection
- Women who are co-infected with high-risk HPV types and HIV have an elevated risk of cervical cancer
What is p14ARF?
- An alternate reading frame protein product of the CDKN2A locus (i.e. INK4a/ARF locus)
- Induced in response to elevated mitogenic stimulation, like aberrant growth signaling from MYC and Ras
- Accumulates mainly in the nucleolus where it forms stable complexes with NPM or Mdm2, and acts as a tumor suppressor by inhibiting ribosome biogenesis or initiating p53-dependent cell cycle arrest and apoptosis
- p14ARF inhibits Mdm2, so p53, then p21 activation, which binds and inactivates cyclin-CDK complexes, which normally promote transcription of genes that carry cell through the G1/S checkpoint of the cell cycle
- Loss of p14ARF by a homozygous mutation in the CDKN2A (INK4A) gene leads to elevated levels in Mdm2 and loss of p53 function and cell cycle control
How many types of HPV have been identified?
At least 70 genetically distinct types
Describe precursor lesions, and provide three examples of their metaplastic evidence.
-
Precursor lesion: term that encompasses several types of entities associated with increased cancer risk
1. Do not inevitably progress to cancer, but are important to recognize b/c some precursor lesions can be detected by screening procedures and treated, reducing the risk of developing cancer - Many precursor lesions arise in chronic inflammation, and can be recognized by presence of metaplasia:
1. Barrett esophagus: intestinal metaplasia of the esophageal mucosa due to GERD
2. Squamous metaplasia of the bronchial mucosa due to smoking
3. Intestinal metaplasia of the stomach due to chronic gastritis
Diagnose this.

HSIL (high-grade squamous intraepithelial lesion)
Is HCV infection treatable to prevent the hepatocellular carcinomas it causes?
Yes (complicated and highly evolving)
What is going on here? How might this progress?

- Simple endometrial hyperplasia
- If untreated, might progress through complex endo hyper, to complex endo hyper with atypia, to endo adenocarcinoma
What is going on here?

- Cervical squamous cell carcinoma invading with irregularly shaped islands of cohesive cells with moderate smooth eosinophilic cytoplasm
What is rhabdomyosarcoma?
- RMS: cancer made up of cells that normally develop into skeletal muscle (rhabdomyocytes)
- Cancer of embryonal cells, so much more common in children, but can devo in adults
- Can start anywhere in the body, even areas that don’t typically have skeletal muscle; most common sites are:
1. Head and neck, urogenital, arms and legs, trunk (chest and abdomen)
What is dysplasia?
- Disordered growth
- Acquired form = cellular atypia & messed up architecture due to mutations in genes regulating cell proliferation, but not enough to be cancer… YET
Provide a classic example of a benign neoplastic precursor lesion. Do benign tumors frequently undergo malignant transformation? Why or why not?
- Classic example: colonic villous adenoma, which if left untreated progresses to cancer in about 50% of cases.
- NO -> most benign tumors rarely undergo malignant transformation (e.g., uterine leiomyomas, pleomorphic adenomas of parotid gland), and others not at all (e.g., lipomas)
- Why many benign tumors have negligible risk of malignant transformation is an unsettled question
1. One possibility is that benign tumors at high risk for malignant transformation possess the cancer-enabling property of genomic instability: high freq of mutations in genome of a cellular lineage. Mutations can incl. changes in nucleic acid seq, chromosomal rearrangements or aneuploidy - NOTE: controversial whether uterine leiomyomas ever become malignant. There are uterine leiomyosarcomas, but may arise de novo
Why is the study of chromosomal changes in tumor cells important? How is this done?
- Genes in vicinity of recurrent chrom breakpoints or deletions very likely to be either oncogenes (e.g. MYC, BCL2, ABL) or TSGs (e.g., APC, RB)
- Certain karyotypic abnormalities have diagnostic value or important prognostic or therapeutic implications, e.g., tests that detect and quantify BCR - ABL fusion genes or their mRNA products essential for diagnosis of CML and used to monitor response to BCR-ABL kinase inhibitors
- Historically, identified via karyotyping, but now cancer cell karyotypes are being reconstructed from deep sequencing of cancer cell genomes
What are the characteristics of initiating chemical carcinogens? Name the two categories.
- . All initiating chemical carcinogens are highly reactive electrophiles (have electron-deficient atoms) that can react with nucleophilic (electron-rich) sites in the cell
- Their targets are DNA, RNA, and proteins, and in some cases these interactions cause cell death -> initiation inflicts irreperable DNA damage that is nonlethal
- Mutated cell passes DNA lesions to its daughter cells
- Two categories: direct acting and indirect acting
What is this?

Bile behind obstructing colon cancer
What is the first step in the diagnosis of cancer? Explain your answer.
-
Discovery: symptoms, signs, screening -> radiology, endoscopy, serum tumor markers (below)
1. CA-125: ovarian
2. CA-19-9: pancreas, gallbladder, bile duct, gastric
3. HCG: choriocarcinoma, testicular
4. AFP: liver, germ cell tumors
5. CEA: colorectal, breast
Which strains of HPV cause benign squamous papillomas (warts)? What about genital warts?

- Benign squamous papillomas: 1, 2, 4, and 7
- Low-risk HPVs, mainly HPV-6 and HPV-11 are associated with genital warts, which have low malignant potential
Describe the nuclei of these cancer cells.

- Cancer cell nuclei show abnormal morphologies (due to epigenetic mechanisms, i.e, histone modification and DNA methylation)
- Ex: hyperchromasia, chromatin clumping, or chromatin clearing (aka, vesicular nuclear chromatin)
How is radiant energy exposure related to cancer?
- Radiant energy, in the form of the UV rays of sunlight or as ionizing radiation, is carcinogenic
- UV light is implicated in the causation of skin cancers
- Ionizing radiation exposure from medical or occupational exposure, nuclear plant accidents, and atomic bomb detonations produces a variety of cancers.
What is this?

Gastric adenocarcinoma?
Why do lung cancers associated with smoking have a 10-fold higher mutational burden (on avg) than lung cancers in non-smokers?
- These excess mutations are strongly skewed toward particular base substitutions known to be caused by carcinogens in cigarette smoke (the proverbial “smoking gun”)
Why has the number of deaths due to uterine cervical carcinoma in the US decreased 75% in the last 50 years?
- Screening with PAP smears
- PAP smears detect cervical dysplasia before it becomes cancer
Name 4 important examples of chronic inflammatory conditions leading to cancer.
- Inflammatory bowel disease (IBS), esp. ulcerative colitis, leading to carcinoma of the colon
- Chronic hepatitis, esp. chronic Hep C, leading to hepatocellular carcinoma
- Chronic pancreatitis -> pancreatic carcinoma
- Chronic cholecystitis -> gallbladder carcinoma
- NOTE: variety of chronic inflammatory diseases, incl. those w/infectious and non-infectious etiologies, can lead to cancer
What % of women with breast cancer present with a breast mass in the interval between mammograms?
30%
Why is the death rate for stomach carcinoma for M/F 7 times higher in Japan than in the US?
- Nearly all of the evidence indicates these geographical differences are environmental, rather than genetic, in origin
1. They include: high dietary nitrates, high dietary salt, and smoking
2. However, the PRIMARY ETIOLOGIC AGENT of gastric cancer is: infection with helicobacter pylori
Provide an example of how the link between chronic inflammation and cancer can have practical implications?
- Diagnosis and effective treatment of Helicobacter pylori gastritis with antibiotics can quell a chronic inflammatory condition that might otherwise lead to the development of a gastric cancer
What are the 5 direct effects of tumors?
- Impingement on adjacent structures
- Obstruction (e.g., of intestine)
- Functional activity (e.g., hormones)
- Surface ulceration (+/- bleeding, ulceration)
- Infarction (+/- rupture)
List some (less important) potential chemical carcinogens.
- Vinyl chloride
- Arsenic
- Nickel
- Chromium
- Insecticides
- Fungicides
- Polychlorinated biphenyls
How are glycoproteins and glycolipids useful for cancer therapy?
- Most tumors express higher than normal levels and/or abnormal forms of surface glycoproteins & glycolipids,
- These may be diagnostic markers and targets for therapy -> CA-125 and CA-19-9 are mucins expressed on ovarian carcinomas and other types of carcinoma; not sensitive or specific enough to be screening tests, but sometimes useful in following the course of therapy
-
MUC-1 expressed on some ovarian/breast carcinomas, is integral mem protein normally expressed only on the apical surface of breast ductal epithelium, relatively sequestered from the immune system
1. In ductal breast carcinomas, sometimes this molecule is expressed in an unpolarized fashion and contains new, tumor-specific carb and peptide epitopes detectable by mouse MAbs
Briefly describe what types of genetic damage can activate oncogenes or inactivate TSGs.
- May be subtle (e.g., point mutations) or involve chrom segments large enough to be detected via karyotype
- Certain chrom abnormalities highly associated with particular neoplasms, & inevitably lead to dysregulation of genes w/integral role in pathogenesis of that tumor type -> specific recurrent chrom abnormalities identified in most leukemias and lymphomas, many sarcomas, and an increasing number of carcinomas
- Whole chroms may be gained or lost; while changes in chrom number (aneuploidy) and structure generally late phenomena in cancer progression, in some cases (e.g., in cells that have lost their telomeres), it can be an early event that initiates the transformation process
What are some examples of abnormally expressed normal cellular proteins that can be tumor antigens?
- Tyrosinase: enzyme involved in melanin biosynthesis expressed only in normal melanocytes and melanomas
-
Cancer-testis antigens: encoded by genes silent in all adult tissues except germ cells in testes -> prototypic of this group is melanoma antigen gene (MAGE) family
1. MAGE-1 expressed on 37% of melanomas and some lung, liver, stomach, esophageal carcinomas. Other members include: RAGE, GAGE, etc.
What are the features of anaplastic cells?

- Larger than differentiated cells
- Higher nuclear/cytoplasmic ratio: bigger nuclei, less cytoplasm
- Pleomorphic (varying in size, shape)
- Nuclear abnormalities: angulated shape, clumped chromatin, hyperchromatism, mitoses, nucleoli
What is this?

- Pituitary adenoma: most common source of ACTH causing Cushing syndrome
- Small cell carcinoma of lung common too
What are the risk factors for breast cancer? Come on, there are only 75 million.
- Family history, older age, race (white > black > asian), and height (being taller)
- Obesity (postmenopausal), eating red meat, dietary fat (maybe), smoking, moderate alcohol, not exercising
- Radiation, AB use, nocturnal light exposure (via suppression of nocturnal production of melanin)
- Early menarche, hormone levels (estrogen and testosterone), late menopause, fewer/later pregnancies, not breast feeding
What types of cancers are associated with H. pylori? How is the bug’s genome implicated?

- Implicated in gastric adenocarcinomas and gastric lymphomas of mucosa-associated lymphoid tissue, or MALTomas (first bacterium classified as a carcinogen)
- Genome has genes directly implicated in oncogenesis
1. Strains associated with gastric adenocarcinoma contain a “pathogenicity island” with cytotoxin-associated A (CagA) gene - While noninvasive, CagA penetrates gastric epi cells, and has variety of effects -> initiates signaling cascade thatmimics unregulated growth factor stimulation
- Note: H. pylori was first ID’d as cause of peptic ulcers
Diagnose this Pap.

HSIL (high-grade squamous intraepithelial lesion)
In addition to chronic liver cell injury and compensatory regeneration, what components of the HCV genome may have a direct effect on tumorigenesis?
- Molecular mechanisms used by HCV less well-defined than those of HBV -> HCV core protein may have a direct effect on tumorigenesis, perhaps by activating variety of growth-promoting signal transduction pathways
- Note: HCV is not a DNA virus, so it cannot undergo viral integration, unlike HBV
Why is lung cancer incidence lower than that of prostate and breast, but its death rate higher?
- Because lung cancer is much more fatal than prostate or breast cancer
What are some things that may be protective in terms of risk of developing colon cancer?
- Evidence supports protective effect of aspirin and other NSAIDs on devo of colonic adenomas and cancer
-
Protective diet:
1. Avoidance of processed and charred red meat
2. Inclusion of veggies (esp. cruciferous), and unprocessed forms of wheat bran (controversial)
3. Adequate amount of intake of folate from food
4. Limited caloric intake and avoidance of excess alcohol
What is the scenario/sequence for the development of gastric adenocarcinoma with H. pylori infection?
- Increased epithelial cell proliferation in a background of chronic inflammation -> the inflammatory milieu has numerous genotoxic agents, like ROS
- Initial development of chronic gastritis, followed by gastric atrophy, intestinal metaplasia of the lining cells, dysplasia, and cancer
- This sequence takes decades, and occurs in only 2-3% of infected patients
What are differentiation antigens?
- Molecules expressed by tumors that are normally present on their cells of origin
- Abs against CD20, transmembrane protein expressed on surface of normal mature B cells, have broad cytocidal activity against mature B-cell lymphomas and leukemias, and are widely used in tx
- MAbs may also be covalently coupled to drugs, toxins, or radiochemicals, so Ab serves as guided missile that delivers therapeutic warhead to cancers expressing particular surface antigens
What is the most common lung cancer type to cause hypercalcemia?
Squamous cell carcinoma
What is this?

Normal endometrium
Why is the epigenome an attractive therapeutic target?
- Because the epigenetic state of a cell depends on reversible modifications that are carried out by enzymes, which are generally good drug targets
- Inhibitors of histone deacetylases, chromatin erasers that remove acetyl groups from histones, are approved for use in certain lymphoid tumors
- DNA methylation inhibitors are now being used to treat myeloid tumors
What is a potential consequence of epigenetic heterogeneity in cancers? Why might this be a concern?
- Cancers may exhibit considerable epigenetic heterogeneity b/c just as genomic instability gives rise to genetic heterogeneity, it is feared cancers will also prove to have extensive epigenetic heterogeneity from cell to cell within individual tumors
- A consequence of such heterogeneity may be drug resistance. For example, epigenetic alterations can lead to resistance of lung cancer cells to EGFR inhibitors, making epigenetic plasticity another barrier to the development of curative cancer therapies


