Carbohydrate Metabolism I Flashcards
Favorable & Unfavorable Processes
- Favorable processes (deltaG is negative2) .
- Unfavorable processes (DeltaG is Positive)
- Coupling of a favorable processes (negDeltaG) with an unfavorable processes (posDeltaG) to yield an overall negative DeltaG
- Endergonic reactions (energy requiring) can be coupled to the exergonic (energy yielding) hydrolysis of ATP.
ATP Hydrolysis
- The change in free energy asssociated with the hydrolysis of ATP is due to the:
a) repulsion of adjacent negatively charged oxygen atoms and
b) the resonance stabilization associated with the products i.e. ADP - ATP + H2O —-> ADP + Pi + ENERGY

ATP Generation
- ADP + Pi –> ATP + H2O
- Requires input of 7.3 kcal per mole of ATP
- ATP can be generated in cytosol (glycolysis)
- ATP can be generated in mitochondria (aerobic respiration) - vast majority of ATP production here.
ATP Consumption
ATP is constantly being consumed:
- ATP hydrolysis ATP + H2O –> ADP + Pi + ENERGY
- Coupling to endergonic reactions
- Active Transport (lower to higher gradient)
- Muscular contractions
- Maintenance of cell volume
- Powering of cilia
- Nerve impulses
- Phosphorylation (enzymatically donating and covalently attaching a phosphate group to a substrate)
- Kinases add phophate group
- Transferase remove phosphate group (and transfer it to the target protein)
- ATP has other roles in the body, including extracellular signaling
- ATP is constantly replenished by the oxidation of foods
ATP Physiological Significance
- Not all biologically significant “high energy compounds” (which btw, ATP is not really) contain a phosphate group, e.g. Acetyl-CoA
- A person engaging in moderate physical activity produces about 60 Kg of ATP per day, while the total human body stores approximately 50 g; even that is later used up in everyday activity.
- 2100 Kcal/7 Kcal (to make ATP) = 300 moles of ATP
- 300 moles (w/mw) = x/500 –> 150,000 g = 150 Kg
- In reality, only 60 Kg ATP (NOT efficient)
- Equilibrium between ATP, ADP, and AMP is maintained by different mechanisms, which are all very critical.
Cellular Reception
Metabolic regulation can be:
-
Intracellular
- Product inhibition
- Allosteric inhibition
- Allosteric activation
- Etc.
-
Intercellular
- Surface to surface cell contact
- Hormones, growth factors, cytokines, neurochemicals, etc.
- Synaptic signlaing between cells transmitting messages
Mechanisms for transmission of regulatory signals between cells
- Synaptic signaling via Neurotransmitters through nerve cell
- Endocrine signaling via hormone through capillaries
- Direct contact via signaling cells through Gap Junctions.
Cells must respond to signals from
- Endocrine (distant locations); glands, hormones via capillaries
- Paracrine (nearby locations); “paracrine factors”
- Autocrine (same cells)
Second Messenger System
- Operate as an amplifying cascade that transmits the binding effect of hormones, growth factors, cytokines, neurochemicals, etc., to a specific cellular response.
- The cell releases second messenger intracellularly in response to exposure to extracellular signals - the first messenger.
- There are a number of different membrane receptors including G Protein-Coupled Receptors (GPCR’s).
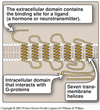
G Protein-coupled receptor
- The extracellular domain contains the binding site for a ligand (a hormone or neurotransmitter).
- Has seven transembrane helices within the lipid bilayer.
- Intracellular domain (tertiary structure) that interacts with G-proteins.
- The act of extracellular signal binding transmits the message into the cell via inducing conformation changes in the receptor.
- These ligands bind with weak forces since at some point they need to get readily detached.
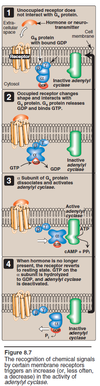
G Protein in action
- Unocccupied receptor does not interact with Gs (stimulation) protein, which is a heteroTrimer with GDP bound.
- Occupied receptor changes shape and interacts with Gs protein. Gs protein releases GDP and binds GTP.
- α Subunit of Gs protein (GTP bound) dissociates and activates adenylyl cyclase, which is responsible for ATP → cAMP + PPi (two phosphates).
- When hormone is no longer present, the receptor reverts to resting state. GTP on the α subunit is hydrolyzed to GDP, and adenylyl cyclase is deactivated.
Various G Alpha subunits
- Gs - stimulates adenylyl cyclase
- Gq - stimulates inositol triphosphate and diacylglycerol
- Gi - inhibits adenylyl cyclase
- Gt - associated with transducin
*No need to know too much in details*
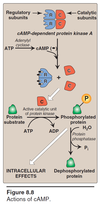
Actions of cAMP
- cAMP-dependent protein kinase A has 2 Regulatory (repressor) subunits and 2 Catalytic (kinase) subunits i.e. heterotetramer.
- ATP —adenylyl cyclase—> cAMP
- 4 cAMP units bind to the Regulatory subunits (2 in each subunit) and Catalytic subunits are dissociated and become active catalytic unit of protein kinase
- Certain protein substrate then gets phosphrylated via ATP hydrolysis carried out by Active catalytic unit of protein kinase, thusly resulting in intracellular effects.
- Protein phosphatase will revert the phosphorylation process and return the protein substrate to original state.
- “Phosphorylatable” amino acids include Serine, Threonine, and Tyrosine in proteins.
- Note that ATP is not only used for energy purposes but also for cellular processes i.e.phosphorylation.
Protein Kinase A (PKA)
- PKA is highly selective
- There are also cAMP-independent protein kinases
- Catalytic subunits phosphorylate serine (S), Threonine (T), and Tyrosine (Y) sidechains.
- Cholera toxin activates adenylyl cyclase in intestinal mucosa, resulting in the loss of salts from the intestinal epithelium followed by osmotically generated diarrhea.
- Pertusis toxin inhibits the inhibition of adenylyl cyclase.
Cyclic Nucleotide Phosphodiesterase
- Cyclic AMP is hydrolyzed to 5’-AMP by cyclic nuclotide phosphodiesterase (PDE); phosphodiester bond = bonds between sugars and phosphate groups
- There are 11 different families of cyclic nucleotide PDE’s: 1, 3, 4, 5, etc.
- There is pharmacological potential in inhibition of different PDE’s, which means increased level of cAMP:
- Methylxanthine derivatives (theophylline and caffeine) not responsible for specific names
- Sildenafil; can lead to melanoma however
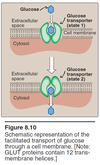
Facilitated Glucose Transporter (GLUT)
- GLUTs allow glucose and other sugars to enter the cell under certain conditions
- There are about a dozen different GLUT (glucose transporter isoforms) including:
- GLUT-1, 3, 4, etc involved in basal glucose uptake from blood and other extracellular fluids. Found in most tissues.
- GLUT-2 is found in liver and kidney cells AND basolaterl membrane of small intestine. It has HIGH Vmax (capacity) and HIGH Km (low affinity); this allows them to be only “really” active when the blood glucose level is high (low affinity) and once needed, get the job done efficiently (high capacity). Both liver and kidney regulate carbohydrate.
- Liver is METABOLIC (e.g. gluconeogenesis, glycogenlysis, & glycogenesis)
- Kidney recycles or excrete excess glucose
- GLUT-5: Transport of fructose
- GLUT-4: Insulin sensitive GLUT found in adipose tissue and skeletal muscle tissue. Insulin (hormone) recruits inactive GLUTs in the Golgi and translocates them to the plasma membrane.
Facilitated transport of GLUT
Facilitated transport
- Glucose binds GLUT
- Direction of GLUT is reversed
- GLUT returns to original state
Sodium Glucose Transport Protein (SGLT)
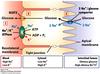
Glucose is transported from LOW concentration (intestinal lumen) to HIGH concentration (epithelial cell) AGAINST the gradient by process of symport or co-transport
- Active transport of Na+ FROM cell into blood creating electrochemical gradient; Na+/K+-ATPase aka sodium-potassium pump; ATPase present since the transport needs energy to power up the pump.
- Na+ enters cell through Sodium Glucose Transport Protein (SGLT) & Glucose is co-transported with Na+; SYMPORT where without glucose available, Na+ does not enter by itself i.e. entry of Na+ is depndent on presence of glucose
- Glucose is transported out of cell, following concentration gradient by GLUT (facilitated passive glucose transport).
- SGLT operates in cells of the intestine, renal tubules, and choroid plexus (brain structure containing cerebrospinal fluid). *no specific name*
- Although SGLT and GLUT don’t use up ATP themselves (both are passive transports), they indirectly requires ATP due to the activity of sodium-potassium pump to create the Na+ gradient in the first place.
Oral rehydration Therapy
- Oral rehydration therapy is predicated on the symport of Na+-Glucose
- Adding glucose into the water help quick hydration along with a pinch of salt.
3.
Blood glucose concetration
- Blood glucose concentration is about 5 mmol/L (about 90 milligrams per 10 liters)
- 180 (MW of glucose) x 0.005 molar x 6 liters of blood = approx. 6 grams of glucose in the blood.
- Very low sugar level in the body meaning it is very efficient in its metabolism/homeostasis.
Carbohydrates
- Name “carbohydrate” is derived from the fact that simple sugars can be represented by the formula (CH2O)n e.g. C6H12O6; “Carbon-Water”
- # C = #O
Carbohydrate Function
- Sources of calories (carbohydrates contain about 4 Kcal per gram)
- Storage of energy (glycogen)
- Bound to proteins and lipids (glycoproteins and glycolipids)
- Structural components e.g. Cellulose
- “Sugar coating” of cells
- Constituents of nucleotides; sugar phosphate backbones of DNA i.e. deoxyribose
Representative human monosaccharides
- 3 Carbons: trioses e.g. Glyceraldehyde
- 5 Carbons: pentoses e.g. Ribose
- 6 Carbons: hexoses e.g. Glucose
- 9 Carbons: nonoses e.g. Neuraminic acid
Alpha Glucose
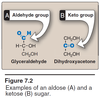
- Aldoses: aldehyde group in the Anomeric Carbon
- Ketoses: keto group in the Anomeric Carbon
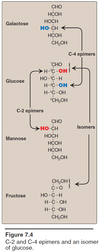
- They have the SAME formula but just different structure e.g. aldose vs ketose; ribose vs ribulose; glucose vs fructose, etc.
- Bottom line they are ISOMERS to each other