Basic Mechanism of Cell Injury, Adaptation, & Cell death Flashcards
List some causes of Cell Injury
- Hypoxia
- Chemicals & Drugs
- Physical Agents
- Microbiologic Agents
- Immunologic Agents
- Genetic Defects
- Nutritional Imbalances
Hypoxia

- Ischemia - Reversible Injury
- Lack of oxygen decreases aerobic respiration (oxidative phosphorylation in mitochondria). Decrease in ATP production. Stimulus to neovascularization
- Acute cellular swelling
- Loss of sodium pumps (Water freely enters the cell)
-
Ribosomal detachment/myelin figures
- Disrupted organelles presenting surface area to hydrophilic cytoplasm
- Na & H2O = freely enters causing cellular swelling & early dilation of ER.
- Dilation can cause ribosomes to detach from RER, reducing protein synthesis
Neovascularization

- Majority of diseases that causes severe vision loss do result of ocular neovascularization secondary to ischemia
- During normal retinal vascular development, vascular endothelial cells proliferate & migrate through extracellular matrix in response to a variety of cytokines leading to formation of new blood vessels in a highly ordered fashion
- During abnormal neovascularization of iris, retina, choroid, angiogenesis (development of new blood vessles) due to ischemia. Causing unregulated and dysfunctional vessels.
- Newly formed vessles can leak fluid, hemorrhage, or associated with fibrous proliferation, retinal edema, retinal/vitreous hemorrhage, retinal detachment. Causing serious vision loss.
Self Perpetuating Chronic Edema

- Thrombosis within a retinal capillary or vein will lead to partial obstruction of blood flow out of the eye, preventing new oxygenated blood entering.
- The subsequent increased intraluminal pressure, if sufficiently high, will cause transudation of blood products into the retina according to starling’s law.
- This will result in increased interstitial (retinal) fluid & protein
- The latter will increase the interstitial oncotic pressure, perpetuating tissue edema, which will impede capillary perfusion & lead to ischemia
Sequela to chronic ischemia: Neovascularization (Diabetic Retinopathy)

- Retinal ischemia from diabetic vascular constriction and dysregulation results in hypoxia, produces enhanced expression of vascular endothelial growth factor (VEGF) and secretion of proinflammatory cytokines (TNF-a, IL-6, and IL-1B). These are the major alterations observed during progression of diabetic retinopathy.
Persistent edema & ischemia lead to _____
- Fibrosis
- Persistent edema will further enhance ischemia leading to TGF -B upregulation and fibrosis
- Note fibrotic ring overlying retinal vascular arcades in proliferative diabetic retinopathy

Available methods to reduce ischemia in the eyes
- injection of anti-VEGF in pars plana every 4-6 wks
- Laster photocoagulation: this purposefully kills off some ischemic retinal tissue, leaving available oxygen for what is left

Persistent ischemia or complete vascular occlusion leads to ____
- infarction: Irreversible Injury
-
Two principle phenomena
- Irreversible mitochondrial dysfunction upon restoration of blood flow
- Cell membrane damage with loss of selective permeability and volume control
-
Calcium influx
- Gap jxn closure/tight jxn release
-
2 types of infarcts
- RED (Venous occlusion)
- WHITE (Arterial occlusion)

What does this image show?

White infarct in the eye - CRAO
What does this image show?

Red infarct in the eye (CRVO)
As in other organ systems, venous occulsion in the eye produces red infract
Venous occlusion releases more fluid & gives rise to hemorrhage more than arterial occlusion
Reperfusion Injury
- Restoration of blood flow to ischemic tissue can promote recovery of cells if they are reversibly injured, but can also worsen injury & cause cell death
- Oxidative stress: new damage may occur during reoxygenation by increased generation of reactive oxygen & nitrogen species
- Intracellular calcium overload: Intracellular and mitochondrial calcium overload begins during acute ischemia and worsens during reperfusion. Calcium overload favors opening of the mitochondrial permeability transition pore with resultant depletion of ATP. This in turn causes further cell injury.
- Inflammation: ischemic injury is associated with inflammation as a result of “danger signals” released from dead cells. This process recruits neutrophils to reperfused tissue. The inflammation causes additional tissue injury
- Activation of complement system: may contribute to ischemia-reperfusion injury. Some IgM antibodies have a propensity to deposit in ischemic tissues. When blood flow is resumed, complement binds to these antibodies, and they are activated, causing more cell injury.
What are some medications that may be toxic to the RPE?
- Direct injury by combining with critical molecule components
-
Toxic affinity for RPE
- Antimalarials (quinine, hydroxychloroquine, mefloquine)
- Phenothiazines (schizophrenia & other psychoses),
- indomethacin (inflammatory)
- Tamoxifen (anti-cancer & prostate)
- Ethambutol optic neuropathy (tx for TB)
-
Toxic affinity for RPE

Mechanism of Chloroquine toxicity
- Hydroxychloroquine binds to melanin and accumulates in the RPE.
- It is directly toxic to RPE = cellular damage & atrophy by disrupting RPE metabolism, specifically from lysosomal damage, and reduced phagocytic activity of shed photoreceptor outer segments.
- Accumulation of undigested photoreceptor outer segments lead to RPE degeneration, migration of RPE cells into the outer retina, & finally photoreceptor cell loss
Chemical injury (Toxic injury)
- Acid & alkali injuries of ocular surface
- Coagulation (acid) vs saponification (base)
- Importance of stem cell preservation

What should you do when either liquid or powder gets into the eye?
- MUST measure pH

What should you do for a pt when a chemical burn to the eye occurs? (according to webMD)
- Flush eye
- Have person put eyes under a faucet, shower, or clean container of wter
- Flush with lukewarm water for 15-30 mins. The person should keep the eye open as wide as possible
- Flush eye to remove CL. Do not try to remove with with hands
- Do not place a bandage over the eye
- Get help immediately
- If you seek medical care (optometrist) . the health care provider will continue flushing the eye with a saline solution (preferably buffered), checking periodically until pH is normal
- The health care provider may place anesthetic drugs in the eye to decrease discomfort with flushing
Acid vs. Alkali
- Acid sears the surface of tissue by coagulating proteins. This coagulation actually inhibits further penetration of acid, thus mitigating further damage once surface pH is neutralized
- Alkali saponifies lipids. even after the surface pH has been neutralized, alkali continues to digest through deeper layers, turning into mush
- Ex. Bleach saponifies skin, feeling greasy and oily between fingers
which is worse, acid or base?
- BASE is worse
- Acid - coagulates protein
- Base - translucent jello & fall apart

Physical agents that can affect the eye?
- Trauma
- Temperature
- Radiant energy
- Oxygen reactive species in the lens

Effects of O2 - Reactive Species
- Light peroxidation of membranes - Polyunsaturated lipids have carbon double bonds that are vulnerable, yielding peroxides that are unstable, leading to a chain reaction of peroxidation and membrane destruction
- Cross-linking of proteins - especially thiol-containing proteins that function as ion pumps
- DNA Damage - impairs protein synthesis/can cause neoplasia
- Mitochondrial Damage - NADPH Depletion/Calcium influx
- Role in cataract formation & ARMD
The Fenton Reaction
Until all of the rust ring around ferrous corneal foreign body been removed with a burr, epithelial healing will not occur
- Fe++ + H2O2 —> . Fe+++ + OH. +OH-
- Transitional metals can accept or donate free electrons can be very toxic because of the generation of the hydroxyl radical OH.
- Aqueous humor has a very high concentration of transferrin to scavenge iron

List some Microbiologic Agents
- Bacteria, their toxins & chlamydia
- Viruses & their antigens
- Fungi
- Protozoan - acanthamoeba, toxoplasma, pneumocystis
- Helminthic - toxocara, onchocerca
- Others (E.g prion disease)
List some immunologic agents
- Complement fixing antibodies
- Anti-nucleoprotein antibodies
- Cytokines
- Immune-complex deposition
Genetic Defects of the eye
- Retinoblastoma - Alteration of a tumor suppressor gene
- Marfan syndrome (Tall & thin)
- Mutation of fibrillin gene (Zonules which are mostly composed of fibrillins can cause ectopia lentis)
Nutritional Imbalances
- Lack of Vitamines (Vital amines)
- A,D,E,K (fat soluble) B’s, C (water soluble)
- Vitamin A deficiency leads to squamous metaplasia of the conjunctiva (bitot’s spot)
- Vitamin K deficiency leads to calcium loss from bone & difficulty with clotting
- B12 deficiency cause anemia because B12 is used to make hemoglobin
- A,D,E,K (fat soluble) B’s, C (water soluble)
- Lack of micro nutrients - trace mineral co-factors
- Decrease in reducing potential - cataract?
- Compromised metabolism/catabolism - drusen?

Cellular Adaptation
- Cells adapt to acceptable changes in their environment by modifying metabolism or growth (e.g. corneal epithelium under a CL) Structural changes
- Increased cellular activity - increase in size or number of cells in response to increased demand
- Decreased cellular activity - reduction in size or number, usually from loss of trophic effect
- Alteration of morphology - change to another normal cell type chosen to optimize survival

Types of cellular adaption
- Atrophy
- Hyperplasia
- Hypertrophy
- Metaplasia
- Dysplasia
- Neoplasia
Describe iris and optic nerve atrophy
-
Iris atrophy
- brown –> greens —> greys –> blue –> bright blue
-
optic nerve atrophy
- loss of axons and reactive gliosis

Hypertrophy/Hyperplasia
- Both lead to greater tissue mass
- Hypertrophy: cell number remains the same and each cell gets larger by adding more cytoplasmic protein
- Hyperplasia: more normal cells are added

Metaplasia
- An adaptive change in which one type of normal cell de-differentiates into a normal but usually less specialized cell type
- If the action is removed the process is reversible
- Conjunctival changes in dry eye
- Chronic posterior uveal inflammation of 10+ years can lead to RPE fibrosis, calcification and squamous metaplastic change in which the RPE is replaced by bone. Some forms of metaplasia can be extreme (and despite the definition), some things that are called metaplasia are NOT reverisble

Dysplasia
- Histological appearance of epithelial cells similar to neoplasia, including increased mitotic activity with incomplete maturation
- An increased nucleus/cytoplasm index is seen & normal architecture can be lost. The provocation is removed, the process is reversible.

Neoplasia cellular features?
- Cellular features of neoplasia
- Increased nucleus/cytoplasm ratio
- Coarse clumping of chromatin
- Increased mitotic figures
- Loss of contact inhibition
- In epithelia, loss of polarity
- Loss of differentiated characteristics or maturation

Adaption resulting from inclusions
- Lipids
- Proteins
- Hyaline changes
- Glycogen
- Pigments (exogenous/endogenous)
Describe Neimann - Pick disease
- Lipid metabolism disorder, where lipid accumulates subcutaneously & taken up by histocytes.
-
Neimann - Pick disease
- autosomal recessive
- No tx - few reach adulthood
- Sx are related to organs in which sphingomyelin accumulates. Enlargement of the liver & spleen
- Cherry red spot in macula due to accumulation of lipids in deep retina
- Accumulation in the CNS & cerebellum can cause ataxia, dysarthria, dysphagia.
- Mutation in the SMPD1 gene cause niemann-pick disease types A & B. The body stops making sphingomyelinase which breaks down the lipid.

Alzheimer’s disease
- Protein misfolding
- Neurofibrillary tangles (red arrow) are formed by hyperphosphorylation of microtubule-associated protein known as tau, causing it to aggregate, or group, in an insoluble form. In Alzheimer’s these are associated with deposits of beta-amyloid (black arrow). Beta-amyloid clumps block cell to cell signaling synapses
Adaptation resulting from inclusion (Hyaline change)
- Amyloidosis is a diverse, heterogenous group of disorders characterized by the depositions of 20+ different forms of amyloid that represent hyaline extracellular material into various tissues throughout the body including the eye & ocular adnexa.
- They result from abnormal protein folding. May involve almost any ocular tissue
- When tissue is stained w/ congo red, amyloid becomes dichroic (birefringent) under polarized light

Adaptation resulting from inclusions (glycogen)
-
Glycogen storage diseases
- usually result from defects in the formation or breakdown of glycogen, primarily in muscles and liver. Rare, with few ocular manifestations
- But abnormal accumulations of glycogen also occurs in diabetes
- Excessive accumulation of glycogen in both layers of the iris epitelium occurs in diabetes. This alters fxn in the cells of both layers - recall that the “myo” component of anterior myoepithelium of iris is the dilator muscle. Hence diabetis often dilate poorly

Adaptation resulting from inclusions - pigments (exogenous)
- The most commonly encountered exogenous pigment is carbon accumulation in the interstices of the lung
- Nowadays, instead of metal salts, organic azo dyes with plastic base pigements are often used in tattooing. These are the materials that are basically used in printing, textile, & car painting industries.
- Causes allergy (Most common)
- Can worsen psoriasis significantly
- Give rise to a lesion histologically indistinguishable from squamous cell CA (Rare)

Adaptation resulting from inclusions - pigments
(endogenous)
- In the time it takes you to read this sentence aloud, roughly 20 million of your red blood cells have died and roughly 5 quintillion (5x1015) molecules of hemoglobin are in need of disposals
- When RBCs break down in the spleen -> hemoglobin is converted into free bilirubin -> Free bilirubin released into plasma which is carried by albumin
- Bilirubin is stripped from albumin by the liver
- Free bilirubin is conjugated to either glucuronic acid or sulfate or it accumulates
- Conjugated bilirubin is secreted into the bile canaliculus as part of bile and thus delivered to the small intestine
- Bacteria in the intestinal lumen metabolize ultimately to sterobilin which gives feces their characteristicis brown color
- Lipofuscin (autofluorescent) - fine yellow-brown pigment granules composed of lipid-containing residues of lysosomal digestion. It is considered to be one of the aging or “wear-and-tear” pigments, found in the liver, kidney, heart muscle, adrenal, nerve cells, and RPE cells
- In the RPE lipofuscin accumulates in lysosmal vacuoles either alone or conjugated with melanin. It is seen in excessive amts in macular degeneration due to inability of RPE cells to digest photoreceptor discs
- Scleral icterus - excessive accumulation of bilirubin (RBC break down product in liver disease)

Injury/Recovery/Cell death (diagram)

Cell death (Necrosis/Apoptosis)
Necrosis
- Coagulation - pale, as if cooked. usually from arterial occlusion
- Liquefaction - semisolid, common arterial occlusion in brain
- Caseous - resembing coarse cream cheese - esp TB
- Gummatous - rubbery consistency - found in syphilis
- Hemorrhagic - Dead tissue suffused with RBCs venous occulsion
- Fat- hard, yellow pattern of adipose cell death
- Fibrinoid - particular to arterial disease with fibrin deposition
What type of necrosis does this show?

Coagulative
What type of necrosis does this show?

Liquifactive (tissue turns into cellular soup)
What type of necrosis does this show?

Caseous
Casein is the principal protein in milk
the tissue loks like curdled milk/cheese. usually seen in context of granulomatous disease, specifically TB.
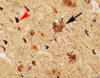
What type of necrosis does this show?

Gummatous - “Gummas” are very rubbery nodules of necrotic tissue seen in tertiary syphilis. This is an unusual case of pulmonary gummas in congenital syphillis
What type of necrosis does this show?

Hemorrhagic
What type of necrosis does this show?

Fat
Common in pancreatitis and in other fatty tissue such as breast
In fat necrosis the enzyme lipase releases fatty acids from triglycerides.
The fatty acids then complex with calcium to form soaps. these soaps appear as white chalky deposits
What type of necrosis does this show?

Fibrinoid
special form of necrosis found primarily in vessel walls caused by immune-mediated vascular damage. It is marked by deposition of antigen & antibodies, sometimes referred to as “immune complexes” within arterial walls together with fibrin - hence the name.
Stage of necrosis
- Pyknosis
- Karyorrhexis
- Karyolysis

Define apoptosis? What are the stages of apoptosis?
- A programmed and energy dependent process designed specifically to switch cells off and eliminate them. This leads to quiet, non-inflammatory cell death & phagocytosis. Numerous initiators can “trip’ the switch

What are the two types of calcification?
-
Dystrophic calcification
- caused by abnormalities or degeneration of tissues resulting in mineral deposition, though blood levels of calcium remain
-
Metastatic
- Deposition of calcium salts in otherwise normal tissue because of elevated serum calcium levels

Choroidal osteoma

- When inflammatory calcification goes on to form bone in the eye
Elevated cytosolic calcium is an important mediator of _____
Cell injury
If membrane damage allows extra calcium to leak in, mitochondria have some capacity to store calcium as granules to keep cytosolic concentrations within tolerable levels

Consequences of elevated cytosolic levels of calcium
- Excessive accumulation in mitochondria is at some cost since it reduces production of ATP
- Increased cystolic Ca++ activates phospholipase that degrade membrane, proteases which destroy essential proteins and calcium undermines junction integrity adhering jxn and closing gap jxn communication
- Increased cytosolic calcium can also lead directly to apoptosis by induction of caspases (initiators and executioners - proteases)
Other mechanisms of cell injury
- Other mechanisms of cell injury
- These & Ca++ influx, rarely occur in isolation since each plays into producing the others
- Depletion of ATP
- Accumulation of Oxygen-derived free radicals
- Defects in membrane permeability
- Damage to DNA & proteins
the depletion of ATP can cause what?
- Commonly associated with hypoxic & toxic chemical injury
- ATP is very important for cellular activity
-
Depletion of ATP by 90+% = cell destruction
- Na, K-ATPase pump shut down -> accumulation of Na -> Swelling & ER shutdown
- Ca fails = Ca influx
- Protein synthesis shuts down
- Ultimately plasma membrane maintenance is compromised
Accumulation of oxygen derived free radicals
- Free radicals - are molecular species that have a single unpaired electron in an outer orbit (e.g. OH-, superoxide and hydrogen peroxide) produced by interaction of cytosal and UV which are by-products of mitochondrial respiration that are normally eliminated using reducing capacity of the cell (NADPH - NADP)
- Oxidative attack on proteins results in site-specific amino acid modifications, fragmentation of the peptide chain, aggregation of cross-linked reaction products, altered electrical charge and increased susceptibility to proteolysis
- Ascorbate fxn as reductant for many free radicals, thereby minimizing the damge caused by oxidative stress
- Ascorbate plays an important role in aqueous and vitreous where its levels are higher than in blood.

Vitrectomy can leave the ___ and ___ to increased oxidative damage (cataract & glaucoma)
lens & TM

Defects in membrane permeability
In ischemic injury, ATP depletion can cause membrane damage via decreased phospholipid synthesis.
Reactive oxygen species give rise to lipid peroxidation
Cytoskeletal abnormalities - Membranes serve to anchor the cytoskeleton especially where modifications of cell shape are critical (e.g. microvilli)
Loss of osmotic control - Na retention, swelling
Damage to DNA and proteins
-
Free radicals (activated oxygen) = damages DNA (deletions, mutations, & other lethal genetic effects)
- Sugar & base moieties are susceptible to oxdiation causing base degradation, SS breakage, & cross-linking protein
- Melanin protects DNA from damage
- You will find a dark pigmented ring around the limbus in those living sunny climates. This helps pigments protects the heavily dividing area from UV damage.

Is tumor growth possible in lens (of eye)?
- No tumor of lens, Despite continous cell division of the lens epithelium and growth of the lens throughout life
- lens epithelium within the pupil is NOT dividing, only under the protection of the iris undergoes mitosis



