Basic Exam Flashcards
What is the half life of Flumazenil?
Why is this important?
Half Life:
Midazolam: 1.7 - 2.6 hours (102 minutes - 156 minutes)
Flumazenil: 0.7 - 1.3 hours (42 min - 78 minutes)
Significantly shorter half life than benzodiazepine agonists.
This can lead to the recrudescence of sedation from benzodiazepines following the elimination of flumazenil.
Therefore, when administering flumazenil, careful attention needs to be paid to its duration of action relative to that of the benzodiazepines taken by the patient, and additional doses of flumazenil should be available.
What is the mechanism of action of Flumazenil?
*Caveat to Mechanism of Action*
Note that although flumazenil is generally considered a benzodiazepine antagonist, it exhibits a partial agonist effect.
In one study, propofol was potentiated by giving high doses of flumazenil, suggesting flumazenil has a mixed or partial agonist effect.
What is the half life of the common benzodiazepines?
Alprazolam, Diazepam, Lorazepam, Midazolam, and Temazepam
Half Life of Other Benzodiazepines (MALTD)
Midazolam is 1.7-2.6 hours (2 hours)
Alprazolam is 6-27 hours (A = Afternoon)
Temazepam is 10 hours (Tem = Ten)
Lorazepam is 11-22 hours (L = eLeven)
Diazepam is 20-50 hours (D = ~ 1 day)
For Nitrous Oxide, a full tank will read:
What volume?
What pressure?
When will the pressure start to drop?
A full tank of N2O contains 1590 L at a pressure of ~745 psig.
Pressure within a tank of N2O will remain at ~745 psig until all liquefied gas is used up which is when the tank is ~16% full (253 L).
What acid base disturbances are common with different diuretics?
Acetazaloamide?
Thiazide?
Loops?
Potassium Sparing?
Thiazide and loop diuretics can cause an Hypokalemic Hypochloremic metabolic alkalosis.
Acetazolamide and potassium sparing can cause a hyperchloremic metabolic acidosis.
How does hyperventilation affect electrolytes?
Calcium?
Potassium?
Phosphate?
Sodium?
pH?
HCO3?
Chloride?
Lactate?
Respiratory alkalosis (ETCO2 of 30-35), such as from hyperventilation, can cause electrolyte abnormalities such as hypocalcemia, hypokalemia, and hypophosphatemia.
Loss of CO2 by 10 mmHg
Rise in pH (0.1)
Decreased HCO3 (2.0 mEq/L)
Decreased Potassium (0.4 mEq/L) - After will slowly return to normal
Decrease in Sodium
Increased Chloride (Balanced decrease in Bicarb)
Increased lactate (Balanced decrease in Bicarb)
What is the relationship between hyper/hypoventilation and calcium levels?
(Explain the physiology)
Hypocalcemia can occur with respiratory alkalosis.
In response to alkalosis, hydrogen ions bound to negatively charged plasma proteins, such as albumin, are released.
Calcium, being positively charged, can then bind to albumin and other proteins decreasing the serum calcium concentration (particularly the free/ionized, active fraction). This is the mechanism behind paresthesias that occur with hyperventilation.

What are the main determinants of myocardial oxygen demand?
1. Wall tension
2. Heart rate
3. Contractility
What is the formula for wall tension?
Wall tension (T) = (P * r) / (2 h)
P is the pressure within the ventricle
r is the radius of the ventricle
h is the thickness of the ventricular wall (Increase seen in hypertrophy)
What is the downside to developing left ventricular hypertrophy?
The ventricle hypertrophies to compensate for the increased wall tension due to pressure or volume overload, such as with aortic stenosis. However, this change comes at a cost. The hypertrophied ventricle is not as compliant due to increased diastolic pressures in the ventricle. This leads to a decrease in preload*, and the hypertrophied ventricle is now more *dependent on atrial contraction to maintain LVEDV
ex: (Atrial fibrillation with diastolic heart failure is a horrible combination)
What are the two determinants of acoustic impedence of ultrasound?
Acoustic Impedance (two factors determine)
1. Density of the medium(This is the main one)
2. Propagation speed of sound through the medium
Acoustic impedance is the product of the density of a medium and the propagation speed of sound through that medium. Ultrasound reflections that occur at the interface of different mediums are due to the changes in acoustic impedance. Since propagation speed changes slightly between biological mediums, acoustic impedance is primarily dependent upon density.
How does gentamicin affect neuromuscular blockade?
What is the mechanism behind this?
Prolongs neuromuscular blockade
Gentamicin inhibits prejunctional acetylcholine release and depresses postjunctional receptor sensitivity to acetylcholine, thus prolonging paralysis in a patient who received it along with a muscle relaxant.
It is believed that aminoglycoside antibiotics antagonize calcium ions by means of its involvement in the process of acetylcholine release by nerve impulses.
What are all the antibiotics that can prolong neuromuscular blockade?
Aminoglycosides
- Lincomycin, Clindamycin, Streptomycin, kanamycin, tobramycin, neomycin, and gentamicin
Polymyxins
Tetracyclines
Muscarinic receptor agonists can act through two mechanisms, directly on the muscarinic receptor or indirectly by inhibiting the breakdown of ACh causing more ACh to be available to bind to the muscarinic receptor.
1. What are the direct acting agents are choline esters?
2. And what are the alkaloids?
3. What are the anticholinergics?
The direct acting agents are choline esters (ACh, methacholine, carbachol, bethanechol)
direct acting agents have few clinical applications due to their very short half and longer activity can be achieved by methylating the choline moiety.
Alkaloids (pilocarpine, muscarine, arecoline or acetylcholinsterase inhibitors).
The indirect acting agents are acetylcholinesterase inhibitors (physostigmine, neostigmine, pyridostigmine, edrophonium, and echothiophate). These medications are often used to improve neuromuscular function in disease states where weakness occurs such as myasthenia gravis or to help reverse the action of nondepolarizing neuromuscular blocking agents.
Anticholinergic drugs are used commonly in anesthesia practice. These medications inhibit the action of ACh by reversibly binding at the muscarinic receptor. Antimuscarinic drugs used in anesthesia practice are atropine, scopolamine, and glycopyrrolate. Both atropine and scopolamine cross the blood-brain barrier, which can result in inhibition of vagal outflow from the central nervous system. At low doses, vagal outflow can potentially be augmented.
You have your line isolation monitor alarming. What is the next step?
When the line isolation monitor alarms, the first step should be to unplug the most recent electronic device that was plugged in.
What portion of the spinal cord is perfused by the anterior spinal artery?
How many are their?
What vessel is this from?
A single (not paired) anterior spinal artery supplies the anterior two-thirds of the spinal cord.
The spinal cord blood supply is anatomically divided into an anterior and a posterior blood supply. The anterior two-thirds of the spinal cord, primarily responsible for motor function, is perfused by the anterior spinal artery (ASA), which originates from the vertebral arteries and receives contributions from various radicular vessels that arise from intercostal arteries.
Is the posterior spinal artery paired?
What is the perfusion to the posterior spinal artery?
What does it arise from?
Two posterior spinal arteries (PSA) stem from the vertebral or posterior inferior cerebellar arteries (PICA). They comprise the blood supply to the posterior one-third of the spinal cord, primarily responsible for sensation, while concurrently receiving intercostal radicular vessel contributions.
What is the origination spinal levels of Artery of Adamkiewicz?
(List the regions from most to least common)
The artery of Adamkiewicz a radicular vessel contributing to anterior spinal cord blood supply
Most commonly originates within the T9 - T12 region
Followed by the L1 - L5 region
least commonly, within the T5 - T8 region
Compare adults vs. infants in terms of:
Spinal cord* vs. *Dural Sac Ending
In adults
Spinal cord ends at about L1-L2 (Conus Medullaris)
Dural sac ends at about S1-S2.
In an infant, these landmarks are shifted slightly caudad (Inferior)
Spinal cord ending at L3-L4
Dural sac terminating at S3-S4
When performing a spinal blockade in an adult, the iliac crest is commonly used as a landmark as it generally corresponds to the level of the L4 interspace is known as What Anatomical Landmark?
Tuffier’s line
What are the 3 drugs that can decrease post-operative delirium with Ketamine administration?
Benzodiazepines, propofol, and barbiturates can decrease ketamine-induced emergence delirium.
An important consideration when using ketamine relates to the high incidence of psychomimetic reactions early in the recovery period. The most common reactions include hallucinations, nightmares, altered cognition, and altered short-term memory. Repeated doses of ketamine may decrease the severity and incidence of emergence delirium, but one of the most effective strategies for prevention of emergence delirium is the use of midazolam approximately five minutes prior to an induction with ketamine.
How does magnesium and Calcium levels affect neuromuscular blockade?
Hypocalcemia potentiates neuromuscular blockade because calcium is important for the release of vesicles containing acetylcholine from nerves at the neuromuscular junction.
When calcium levels are low, less acetylcholine is released.
With less acetylcholine being released there is less competition at the acetylcholine receptor and neuromuscular blockers have a greater effect.
Hypermagnesemia acts similarly by blocking calcium from entering alpha motor neurons and preventing the release of some of the acetylcholine containing vesicles.
How do anti-convulsants affect neuromuscular blockade?
- *Shorten Non-Depolarizing Blockade:**
- Anticonvulsants (e.g. phenytoin, carbamazepine)
What effect does Ketamine have on neuromuscular blockade?
Prolongs
What is the order or potentiating volatile anesthetics for prolonging neuromuscular blockade?
Why is this physiologically seen?
Potentiation from greatest to least for volatile anesthetics is:
desflurane* > *sevoflurane* > *isoflurane.
Desflurane is a volatile anesthetic with a low blood gas partition coefficient of 0.42.
- The low blood gas coefficient means that very little desflurane is dissolved in tissues, but that desflurane is able to rapidly equilibrate with tissues resulting in quick onset and termination of effects.
- This rapid equilibration into tissues including muscle is the reason why desflurane potentiates neuromuscular blockade more than other volatile anesthetics.
- The location of potentiation is likely at the neuromuscular junction and occurs in a dose dependent fashion.
Mechanical ventilation can be categorized by what three variables? Explain them
Ventilators need “TLC”
Mechanical ventilation can be categorized by three variables
1. Trigger - What initiates the breath and depends on ventilatory drive
2. Limit - Governor of PPV and depends on ventilatory requirements related to how much flow* and *volume are required to satisfy metabolic demands
- Cycle - What terminates the inspiratory phase and depends on the duration and ratio of inspiratory time to total breath cycle duration.
How does Morphine improve Coronary Perfusion?
Morphine improves coronary perfusion through a reduction in preload and a reduction in end-diastolic pressures (EDP) in the ventricles.
The EDV is proportional to the EDP generated within the ventricle.
The RV is perfused during both systole and diastole whereas the LV is perfused during diastole only.
Right-sided coronary perfusion pressure during systole is = (aortic systolic pressure - RVEDP).
Left-sided coronary perfusion pressure is the difference between (aortic diastolic pressure and left ventricular end-diastolic pressure (LVEDP)).
Coronary perfusion pressure is improved by REDUCING both RVEDP and LVEDP.
What is the first symptom during central nervous system toxicity of local aneshetics?
Central nervous system toxicity is first noted by circumoral and tongue numbness.
Visual and auditory disturbances (tinnitus) then follow.
The clinical scenario then progresses to dizziness/lightheadedness, muscular twitching, unconsciousness, and seizure activity. These clinical signs may then be followed by cerebral edema, increased intracranial pressure, coma, respiratory arrest, and ultimately cardiovascular depression and death. Patients who are premedicated with anticonvulsants, such as benzodiazepines or barbiturates, may have masked CNS symptoms and develop cardiovascular depression before other signs are apparent.
Areas of regional anesthesia from highest to lowest vascularity include (List the order of greatest to least)
intravenous
tracheal
intercostal
caudal/paracervical
epidural
brachial plexus
sciatic/femoral
spinal
subcutaneous
Intercostal > Caudal > Epidural > Brachial Plexus > Lower Limbs > Sub-Q “IT - ICE BaLLS” is the mnemonic
Rank the Areas of regional anesthesia from highest to lowest vascularity.
Areas of regional anesthesia from highest to lowest vascularity include:
intravenous > tracheal > intercostal > caudal/paracervical > epidural > brachial plexus > sciatic/femoral > spinal > subcutaneous.
What is the possible consequence of using lidocaine through spinal?
Lidocaine has been linked to cauda equina syndrome, causing urinary and fecal incontinence as well as gait disturbances.
This association has been documented in the setting of high concentration (5%) intrathecal lidocaine administered via narrow lumen (e.g. 27 gauge) catheters. The slow administration of 5% lidocaine through these catheters allows for pooling of the drug in the region of the cauda equina. This form of local neural toxicity may be due to demyelination.
If you have a desiccated CO2 absorbent, what is the byproduct of desflurane?
Of sevoflurane?
Carbon monoxide and heat are produced from the degradation of anesthetic agents in the presence of desiccated carbon dioxide absorbent. Among today’s volatile anesthetics, degradation of desflurane produces the most carbon monoxide.
Sevoflurane = Compound A
When would you see a Saphenous Nerve injury?
Where is the symptoms anatomically?
Injury to a saphenous nerve can cause numbness, pain, and/or paresthesias along the medial lower leg.
Perioperative saphenous nerve injury is relatively uncommon but, due to its close proximity to the great saphenous vein, is a known complication of saphenous vein harvest (e.g., vein graft harvest for coronary artery bypass grafting) or saphenous vein stripping.
Rarely, saphenous nerve block can also cause temporary or permanent nerve injury.
Damage to the saphenous nerve may result in temporary or permanent cutaneous sensory loss, pain, or paresthesias.
Anatomy:
Major sensory branch of the femoral nerve that is primarily responsible for cutaneous sensation of the medial lower leg
What does the Sciatic Nerve split into at the area of the knee?
Tibial nerve
Common Peroneal nerve

What does the superficial peroneal nerve innervate (Include MOTOR and SENSORY)?
Mixed sensory and motor nerve that innervates the peroneus longus and peroneus brevis muscles (which allow foot eversion)
Provides sensation to the lateral lower leg and most of the dorsum of the foot.

When are common peroneal nerve injuries seen?
Common peroneal nerve injury is the most common isolated mononeuropathy of the lower extremities.
Perioperative injuries can occur due to knee hyperflexion (e.g., during lithotomy position in stirrups or boots).
Excessive hip flexion leading to a sciatic nerve stretch injury can also damage nerve fibers prior to their division into the common peroneal nerve.

What is the movement responsible for deep peroneal nerve?
What is the sensory innervation of deep peroneal nerve?
What type of injury, and symptom is common from deep peroneal nerve injury?
Mixed sensory and motor nerve that innervates many major lower leg muscles which are primarily responsible for foot dorsiflexion.
Provides cutaneous sensation to a small patch of skin between the first and second toes.
Isolated injury to this nerve is most commonly caused by trauma to the lateral knee and typically leads to foot drop.

Tibial Nerve
Branch of what nerve?
What movement does this control?
The tibial nerve is a motor and sensory branch of the sciatic nerve.
It innervates the lower leg posterior compartment muscles primarily responsible for foot plantar flexion.
The tibial nerve also provides cutaneous sensation to the posterolateral lower leg and lateral foot.
Tibial nerve injury is most commonly caused by trauma to the lower leg or ankle.

Expected changes in pH, HCO3, and PaCO2 in:
Acute Respiratory Acidosis
Chronic Respiratory Acidosis
Chronic Respiratory Alkalosis
Acute respiratory acidosis should demonstrate a pH decrease of 0.05 and an HCO3- increase of 1.0 mEq/L per acute 10 mm Hg increase in PaCO2.
Chronic respiratory acidosis, pH nearly normalizes, and HCO3- concentrations increase 4-5 mEq/L per 10 mm Hg sustained increase in PaCO2.
Respiratory alkalosis becomes chronic, pH nearly normalizes and HCO3- decreases 5-6 mEq/L per 10 mm Hg sustained decrease in PaCO2.
What is the most common variant of pseudocholinesterase deficiency?
Approximately 20 genetic variants of the BCHE gene exist with the A- and K-variants being the most common.
What medicine is an anticholinesterase used to treat refractory glaucoma by causing miosis?
Since it inhibits BCHE, systemic absorption can cause up to a 95% decrease in BCHE function, thereby potentiating the effects of succinylcholine.
Echothiophate
What are the 4 phases of diastole?
There are four phases during diastole:
Isovolumetric relaxation: this phase begins with the closure of the aortic valve and continues till the opening of the mitral valve. During this time, the left ventricle is relaxing, however there is no change in the ventricular volume (isovolumetric).
Early rapid filling: this phase begins with the opening of the mitral valve. The left ventricle begins to fill with blood from the left atrium. The flow of blood is driven by the transmitral pressure gradient. This phase contributes the largest volume of blood to the left ventricle during diastole.
Diastasis (slow filling): as the left ventricle fills, the pressure difference between the left ventricle and atrium decrease. This slows down the filling considerably, and contributes approximately 5% of the preload. This phase occurs mid-diastole.
Late rapid filling (atrial contraction): the left atrium contracts, ejecting additional blood into the left ventricle. This phase can contribute 15-20% of the preload. Atrial contraction is the last phase of diastole. The mitral valve closes after the atrial contraction.
Why does the FA/FI curve rise faster for nitrous oxide compared to desflurane?
The absorption of nitrous oxide is augmented by the concentration effect, making the rate of absorption faster than desflurande despite their similar blood gas partition coefficients.
What are the 3 forms in which CO2 is transported?
Carbon dioxide is transported in the blood as:
1. Dissolved CO2
Carbon dioxide is 10 times more soluble in blood than oxygen: 0.031 mmol/L/mm Hg versus 0.003 mmol/L/mm Hg, respectively.
2. Bicarbonate *Majority*
This occurs because red blood cells and vascular endothelium contain carbonic anhydrase, an enzyme used to convert carbon dioxide to bicarbonate. The enzyme also catalyzes the reverse reaction. In either case, a transient intermediate, carbonic acid, is created.
Bicarbonate production: H2O + CO2 ↔ H2CO3 ↔ H+ + HCO3-
3. Carbamino compounds.
Carbamino compounds also transport carbon dioxide in the blood. Carbamino compounds are produced from a reaction with proteins. The small amount of CO2 transported in this manner mostly interacts with hemoglobin proteins and to a lesser extent plasma proteins.
Carbamino compound production: R-NH2 + CO2 → R-NH-CO2- + H+
Which blood product is most likely to cause transfusion related sepsis?
Transfusion-associated sepsis is the third leading cause of transfusion-related deaths in the United States and is most commonly caused by bacterial infection from contaminated platelets.
Unlike other blood products, platelets are stored at room temperature which leads to increased bacterial growth.
What urine to plasma osmolar ratio indicates pre-renal oliguria?
A urine-to-plasma osmolar ratio (UOSM : POSM) >1.5 indicates prerenal oliguria (generally secondary to hypovolemia).
The UOSM : POSM is used to assess the tubular response of the kidneys to dehydration or hypovolemia. In this setting of prerenal oliguria, this formula evaluates the kidneys’ ability to retain Na+ and water and produce highly concentrated urine by increasing urine osmolality above 450 mOsm/kg.
By comparison, normal plasma osmolality is 280-300 mOsm/kg.
The UOSM : POSM indicates the kidneys’ ability to concentrate urine. Tubular damage and acute renal failure, therefore, may be represented by a decreased ratio.
The UOSM : POSM can also decrease with the administration of diuretics. As an aside, isosthenuria is a UOSM : POSM equal to 1.
MOA of:
Hydrochlorothiazide?
Furosemide?
Spirinolactone?
HCTZ = It works by blocking the Na/Cl co-transporter in the DCT
This causes inhibition of sodium reabsorption in the distal convoluted tubules, thereby increasing the excretion of both sodium and water.
Furosemide = Na/K/Cl in Loop of Henle Blocked
Spirinolactone = Blocks aldosterone receptor in DCT
Which of the following findings on physical examination is generally considered the BEST predictor for difficult tracheal intubation in morbidly obese patients?
Three factors obtained by history or physical exam that correlate with difficult intubations in obese patients include
1. Increased neck circumference **Best single indicator**
2. Mallampati class III or IV airway
3. Presence of obstructive sleep apnea.
In general, increased age, male sex, TMJ pathology, and abnormal upper teeth also correlate with difficult intubations.
What are the common chemo toxocities for?
Cisplatain, Carboplatin?
Acoustic nerve damage
Nephrotoxicity
Chemotoxicity for:
Vincristine
Vincristine: peripheral neuropathy
Chemotoxicity for:
Bleomycin, Busulfan
Pulmonary fibrosis
Chemo toxicity for Doxorubicin?
Cardiotoxicity
Chemo toxicity for Trastuzumab?
Cardiotoxicity
Chemo toxicity for Cyclophosphamide?
Hemorrhagic Cystitis
Chemo toxicity for 5-FU, 6-MP, methotrexate?
myelosuppression
What are the contraindications to succinylcholine therapy?
BRIGMS(S) “Brigham’s”
Burns
Relaxants - Chronic
Immobility (Prolonged)
Guillain-Barre Syndrome
MS
Stroke & Spinal Cord injury
Spinal cord injury
What are the 5 triggers for vomiting?
The vomiting center is stimulated by:
1) the chemotactic trigger zone (CTZ) located in the medulla
2) gastrointestinal tract
3) pharynx
4) visual centers
5) mediastinum
What is the onset time of Scopalamine patch?
What are the side effects of it?
Anticholinergics (e.g., scopolamine patch):
Needs to be applied prior to going back to the operating room due to its 2 to 4 hour onset time.
Can cause visual changes, dry mouth, and dizziness.
Droperidol:
Class?
Mechanism?
Dose?
Dopamine-2 receptor antagonist (e.g., droperidol, metoclopramide): droperidol is effective as an anti-emetic.
Recommended dosing is 0.625 to 1.25 mg at the end of surgery
What are the independent criteria for:
MELD Score?
vs.
Child’s Pugh?
Mnemonics to help differentiate MELD from Child-Pugh:
MELD: “I Crush Several Beers Daily” for INR, creatinine, sodium, bilirubin, dialysis
Childs-Pugh: “Pour Another Beer At Eleven” for PT, Ascites, Bilirubin, Albumin, Encephalopathy
What is a normal Strong Ion Difference?
What is the equation?
Normal human plasma has a SID of 40-44 mEq/L.
SID = ( [Na+] + [K+] + [Ca2+] + [Mg2+] ) - ( [Cl-] + [A-] )
Plasma pH Theories are determined by what 3 independent factors?
The concept of SID proposes that plasma pH is determined by three independent factors:
1. PCO2
2. SID
Strong ion difference represents the difference between the charge of plasma strong cations (sodium, potassium, calcium, magnesium) and anions (chloride and other strong anions (A-) such as lactate, sulfate, ketoacids, and nonesterified fatty acids).
3. Atot.
The latter (Atot) represents the total plasma concentration of nonvolatile buffers (e.g., albumin, globulins, and inorganic phosphate).
Large volume saline:
What happens to:
1. Bicarbonate?
2. pH?
3. SID?
4. Anion Gap?
5. Chloride level?
Large volumes and rapid administration of normal saline can produce a non-anion gap hyperchloremic metabolic acidosis. Associated laboratory values include decreased plasma HCO3-, increased plasma Cl-, decreased SID, and a normal anion gap.
Why is subendocardial ischemia seen more commonly than epicardial transmural injury?
Subendocardial ischemia is more commonly seen than transmural injury because the small capillaries and arterioles at the subendocardial level are subject to occlusive high intraventricular pressure.
The epicardial coronary arteries in comparison are distant from the high intraventricular pressures and thus generally unaffected unless acute occlusion from a thrombus, spasm, or embolism occurs.
For ST Elevation, what are your mV criteria and lead criteria for men and women, respectively?
≥ 0.1 mV in all leads other than leads V2–V3 where the following cut-points apply:
≥ 0.2 mV in men > 40 years
≥0.25 mV in men < 40 years
≥ 0.15 mV in women
What is your criteria for ST depression? and T wave inversions regarding mV and also R/S ratio?
New horizontal or down-sloping ST depression ≥ 0.05 mV in two contiguous leads and/or
T inversion ≥ 0.1 mV in two contiguous leads with prominent R wave or R/S ratio > 1
What are the sensitivities of EKG lead monitoring during surgery?
Recent studies have shown that the most sensitive lead for detection of myocardial ischemia in patients who actually had an MI is lead V4 (sensitivity of 83%) but when two precordial leads are monitored simultaneously the sensitivity increases to 97%.
A separate study suggested that adding lead II with two precordial leads increases the sensitivity for detection of myocardial ischemia to 98%.
What is the correlation between burn patient’s and neuromuscular blockers?
Which are contraindicated?
Which are not-affected (minimally)?
Patients with massive burns demonstrate resistance to NDNMBs, resulting in increased dosing requirements, due to upregulation of ACh receptors and increased plasma protein binding.
Contraindicated = Succinylcholine
Mivacurium’s dosing requirements are only slightly increased relative to other NDNMBs as it is metabolized by pseudocholinesterase, the levels of which are decreased in burn patients.
What is the pathophysiological reason that burn patient’s have increased requirements to non-depolarizing neuromuscular blockers?
Since NDNMB’s drug effect is produced by competitively inhibiting ACh receptors, the presence of more receptors means a higher dose of free drug is required to produce neuromuscular blockade manifesting as twitch depression.
Burn patients also have increased protein binding, meaning less free drugs are available to bind ACh receptors, further increasing dose requirements.
How do you adjust the doses of neostigmine and glycopyrolate for ESRD or CKD patients?
The durations of action of commonly-used anticholinesterases and anticholinergic drugs for reversal of nondepolarizing neuromuscular blockade are prolonged in the setting of CKD and ESRD.
However, no dosage alterations are required and the normal maximum recommended doses still apply.
What is the unfortunate effects of increasing your anti-cholinesterase dose for neuromuscular blockade reversal?
Increasing the dose of anticholinesterase relative to anticholinergic drug:
- Increases the risk for gastrointestinal upset
- Nausea/vomiting
- Cardiac muscarinic effects including significant bradycardia or other arrhythmias.
What is the unintended effect of increasing your anti-cholinergic relative to your anti-cholinesterase?
Increasing the dose of anticholinergic relative to anticholinesterase:
- Increases the risk for tachycardia and other arrhythmias
- Blurred vision
- Dry mouth
Confusion or hallucination may be seen with anticholinergics that cross the blood-brain barrier (e.g. atropine).
At what dose of methadone is there a significant increased risk of QT prolongation?
The risk for prolonged QT intervals is greatest in patients taking greater than 120 mg daily, however, lower doses and acute use can still prolong the QT interval.
Because methadone is metabolized by cytochrome p450, drugs inhibiting this enzyme may lead to increased methadone toxicity and further QT prolongation.
What is the mechanism for which Etomidate causes adrenal suppresion?
It causes dose-dependent inhibition of adrenal mitochondrial 11β-hydroxylase.
The enzyme 11β-hydroxylase is responsible for the conversion of 11-deoxycortisol to cortisol.
Thus, administration of etomidate causes suppression of adrenal cortisol synthesis.
What is the relationship between Pulmonary Vascular Resistance and Lung Volumes?
What is the reasoning behind this?
Pulmonary vascular resistance is affected by lung volumes.
It is lowest at FRC while increasing or decreasing lung volumes beyond FRC results in an increase in PVR.
Highest at Residual Volume and Total Lung Capacity
Pulmonary vascular resistance increases as lung volumes increase due to compression of the smaller pulmonary vessels, notably the small arterioles. As lung volumes increase, alveoli expand and the transmural (distending) pressure in and radii of the small blood vessels decrease. Therefore, PVR increases. Recall that resistance (R) is inversely proportional to vessel radius (r) to the fourth power (R ∝ 1/r4).
Pulmonary vascular resistance increases as lung volumes decrease due to what two mechanisms?
First, as the size of the alveoli decrease and especially when they collapse, the geometry of the pulmonary vessels surrounding these alveoli changes such that the vessels can essentially become kinked, resulting in a significant increase in resistance to flow.
Second, as lung volumes decrease below normal, the volume of blood in the larger pulmonary vessels decreases. This suggests a likely decrease in vessel radius and therefore increased resistance to flow.
What are the two most important factors for release of Vasopression* aka *ADH* aka *Arginine Vasopressin?
- Hyperosmolality (milliosmoles per kilogram. A normal result is typically 275 to 295 milliosmoles per kilogram)
- Reduced Effective Circulatory Volume
What is the mechanism of action of Vasopressin?
Water Balance:
Stimulates water reabsorption by increasing intracellular levels of cyclic adenosine monophosphate (cAMP) and activating protein kinase A
Vasoactive
Vasoconstriction by activating G protein and phospholipase C, which releases calcium from the sarcoplasmic reticulum. There are two classes of AVP receptors:
V1 receptors present in vascular smooth muscle
V2 receptors present in the distal and collecting tubules of the kidney
Transtracheal injection of local anesthetic will block which nerve?
Transtracheal injection of local anesthetic will block the recurrent laryngeal nerve

How long can FFP be stored after being thawed?
Why is this?
FFP is frozen for storage. It can be transfused up to 5 days after thawing
Factors VIII and V are the least stable factors in blood products and can degrade above 4 degreres Celcius.
Significant reductions in factor VIII (41 ±8%) and factor V (66 ±9%) levels.
What are the two opposing processes that determine a drug’s context sensitive half time?
- Plasma clearance
- Redistribution
What is diastasis and when does it occur?
Diastasis occurs when there is complete left ventricular relaxation and filling of the ventricle eventually slows down.
It occurs* during the *filling portion following isovolumetric relaxation.
The spead of local anesthetic in the intrathecal space is primarily determined by what 2 factors?
- Baricity of the local anesthetic solution
(Baricity refers to the density of a substance compared to the density of human cerebrospinal fluid)
Solutions that have a baricity approaching 1.000 are referred to as isobaric*, as the density of the cerebrospinal fluid is approximately 1.0003+/- 0.0003. Solutions with a baricity less than 0.999 are termed hypobaric, and are usually created by mixing the local anesthetic with distilled water. *Hyperbaric solutions are created by mixing dextrose 5-8% with the desired local anesthetic.
Hyperbaric solutions will flow in the direction of gravity and settle in the most dependent areas of the intrathecal space. Conversely, hypobaric mixtures will rise in relation to gravitational pull.
2. Patient position.
You have a patient with suspected opioid induced biliary colic, what are your drug options?
1. Atropine (Blocks parasympathetic activation)
2. Papaverine - opium alkaloid antispasmodic drug, used primarily in the treatment of visceral spasm and vasospasm (especially those involving the intestines, heart, or brain
3. Naloxone - (blocking opioid receptor activation)
How does the ETT depth become affected with neck flexion vs. neck extension?
Neck flexion - Risk of endobronchial intubation
Neck extension - Risk of ETT herniating out of the larynx.
What are the changes in ESRD disease in regard to:
Calcium levels?
Potassium levels?
Magnesium levels?
Lipid Panel?
Phosphate Levels?
- *- Hypocalcemia
- Hyperkalemia
- Hypermagnesemia
- Hyperlipidemia
- Hyperphosphatemia**
With Citrate Toxicity, what is the expected change in Calcium levels?
in Magnesium levels?
Citrate Chelates both magnesium and calcium so you have:
Hypomagnesemia and Hypocalcemia
Majority of Citrate is found in what blood products?
FFP and Platelets
(NOT PRBC)
What is the makeup of D5W in terms of:
Osmolarity (mOsm/L)
Sodium (mEq/L)
Chloride (mEq/L)
Potassium (mEq/L)
Glucose (g/L)
Lactate (mEq/L)
Osmolarity (mOsm/L) 253
Sodium (mEq/L) None
Chloride (mEq/L) None
Potassium (mEq/L) None
Glucose (g/L) 50 grams / Liter
Lactate (mEq/L) None
What is the makeup of D5 1/4 NS in terms of:
Osmolarity (mOsm/L)
Sodium (mEq/L)
Chloride (mEq/L)
Potassium (mEq/L)
Glucose (g/L)
Lactate (mEq/L)
Osmolarity (mOsm/L) 355
Sodium (mEq/L) 38.5
Chloride (mEq/L) 38.5
Potassium (mEq/L) None
Glucose (g/L) 50
Lactate (mEq/L) None
What is the makeup of NS in terms of:
Osmolarity (mOsm/L)
Sodium (mEq/L)
Chloride (mEq/L)
Potassium (mEq/L)
Glucose (g/L)
Lactate (mEq/L)
Osmolarity (mOsm/L) 308
Sodium (mEq/L) 154
Chloride (mEq/L) 154
Potassium (mEq/L) None
Glucose (g/L) None
Lactate (mEq/L) None
What is the makeup of D5 1/2 NS in terms of:
Osmolarity (mOsm/L)
Sodium (mEq/L)
Chloride (mEq/L)
Potassium (mEq/L)
Glucose (g/L)
Lactate (mEq/L)
Osmolarity (mOsm/L) 432
Sodium (mEq/L) 77
Chloride (mEq/L) 77
Potassium (mEq/L) - None
Glucose (g/L) 50
Lactate (mEq/L) - None
What is the makeup of 3% Hypertonic Saline in terms of:
Osmolarity (mOsm/L)
Sodium (mEq/L)
Chloride (mEq/L)
Potassium (mEq/L)
Glucose (g/L)
Lactate (mEq/L)
Osmolarity (mOsm/L) 1026
Sodium (mEq/L) 513
Chloride (mEq/L) 513
Potassium (mEq/L) None
Glucose (g/L) None
Lactate (mEq/L) None
What is the makeup of LR in terms of:
Osmolarity (mOsm/L)
Sodium (mEq/L)
Chloride (mEq/L)
Potassium (mEq/L)
Glucose (g/L)
Lactate (mEq/L)
Osmolarity (mOsm/L) 273
Sodium (mEq/L) 130
Chloride (mEq/L) 109
Potassium (mEq/L) 4
Glucose (g/L) 0
Lactate (mEq/L) 28
What is the makeup of D5LR in terms of:
Osmolarity (mOsm/L)
Sodium (mEq/L)
Chloride (mEq/L)
Potassium (mEq/L)
Glucose (g/L)
Lactate (mEq/L)
Osmolarity (mOsm/L) 525
Sodium (mEq/L) 130
Chloride (mEq/L) 109
Potassium (mEq/L) 4
Glucose (g/L) 50
Lactate (mEq/L) 28
What is the makeup of Plasmalyte in terms of:
Osmolarity (mOsm/L)
Sodium (mEq/L)
Chloride (mEq/L)
Potassium (mEq/L)
Glucose (g/L)
Lactate (mEq/L)
Osmolarity (mOsm/L) 294
Sodium (mEq/L) 140
Chloride (mEq/L) 98
Potassium (mEq/L) 5
Glucose (g/L) None
Lactate (mEq/L) None
What is the makeup of D5NS in terms of:
Osmolarity (mOsm/L)
Sodium (mEq/L)
Chloride (mEq/L)
Potassium (mEq/L)
Glucose (g/L)
Lactate (mEq/L)
Osmolarity (mOsm/L) 586
Sodium (mEq/L) 154
Chloride (mEq/L) 154
Potassium (mEq/L) None
Glucose (g/L) 50
Lactate (mEq/L) None
How would you summarize Boyle’s Law?
P1V1 = P2V2
“Water Boyle’s at a constant temperature”
How would you summarize Charles Law?
V1/T1 = V2/T2
“Prince Charles is under constant pressure to be king”
How would you summarize Gay-Lussac’s Law?
P1 / T1 = P2 / T2
A Culdissac “LUSSAC” has a constant volume and mass of gas
How would you summarize Henry’s Law?
C = kP
Henry’s law indicates that at a constant temperature, the concentration of a gas dissolved in a solution is directly proportional to the partial pressure of that gas: C = kP (where k is a solubility constant) or C ∝ P. As the volume percentage of a volatile anesthetic is increased, the alveolar partial pressure increases.
Example: An increased alveolar partial pressure, therefore, leads to an increased concentration of the volatile anesthetic in the blood which increases the speed of induction and depth of anesthesia.
How would you summarize Dalton’s Law?
P total =- P1 + P2 + P3
Dalton’s law (also called Dalton’s law of partial pressures) states that in a mixture of non-reacting gases, the total pressure exerted is equal to the sum of the partial pressures of the individual gases.
Why is the Desflurane vaporizer heated?
Though it vaporises very readily, it is a liquid at room temperature.
Desflurane vaporizers are typically heated to 39 °C which creates a constant desflurane vapor pressure of 2 atm within the vaporizer, independent of barometric pressure.
What is the maximum dose of neostigmine?
When is the onset/peak effect of neostigmine?
The maximum dose of neostigmine is 70-80 mcg/kg at which point a “ceiling effect” takes place.
This dose results in complete blockade of the acetylcholinesterase enzymes and occurs in approximately 10 minutes.
What is the potential downside to giving additional anticholinesterase (neostigmine) after max dose has already been given?
In some instances additional anticholinesterase medication may cause paradoxical weakening.
The anticholinesterase effect increases the amount of acetylcholine release. In an overdose, depolarization of the endplate caused by the excess acetylcholine can lead to a depolarization block, similar to that seen with succinylcholine.
What are the contraindications to ACE-Inhibitors?
- Renal Artery Stenosis
- Pregnancy
- History of angioedema (whether related to ACE-I or not).
When would you observe a sudden ETCO2 drop to absolute zero?
Circuit Disconnect
Esophageal Intubation
Kinked ETT
When would you observe a drop to ETCO2 to just above zero?
Circuit Leak
Partial Airway Obstruction
What would result in a rapid decrease in ETCO2 but not zero?
Pulmonary embolism
Circulatory Collapse
Would hypothermia cause an increase or decrease in ETCO2?
Decrease
What are some etiologies of gradual increases in ETCO2?
1. Decreased Minute Ventilation
2. Faulty Unidirectional Valve
3. Soda Lime Exhaustion
4. Insufficient Fresh Gas Flow
5. Laparoscopes are inserted into the subcutaneous space causing ETCO2 in the skin (Subcutaneous emphysema)
6. Malignant Hyperthermia
7. Thyroid Storm
How is extravasation of vasopressors best treated?
Keep IV catheter in place initially.
Aspirate about 5 mL of blood to remove as much of the drug as possible.
1. Limb elevation
2. Warm compresses (for vasopressors, cold compress for other drugs)
3. Irrigating with saline (Gault technique)
The Gault technique can be used to wash out the extravasated drug. It starts with injecting hyaluronidase (about 1500 units) into the subcutaneous tissue of the affected area. Four incisions are made at the site and saline (approx. 500 ml) is flushed through one of the incisions. This will irrigate the subcutaneous tissue and flow out the other three incisions.
Hyaluronidase is an enzyme that breaks down hyaluronic acid found in the tissue. This allows the subcutaneous tissue to become more permeable. It is typically used for vesicants, particularly in children (e.g., infant with extravasated parenteral nutrition)
4. Injection of phentolamine
Phentolamine causes the smooth muscles of blood vessels to relax and produces vasodilation. Recommended dose is 5-10 mg within 12 hours of the extravasation. Phentolamine is typically diluted to 0.5 mg/mL then injected in multiple locations, in 1 mL increments, around the border of the extravasation site.
5. Stellate ganglion block (for upper limbs).
In situations with extravasated vasopressors, a stellate ganglion block will create a sympathectomy causing vasodilation in the upper limb.
What is the timeframe in which vasopressor extravasation must be treated?
What is the definitive treatment?
Mild extravasation can lead to local necrosis and more severe extravasations may require open surgical debridement/fasciotomies. (Definitive treatment)
Extravasated vasoconstrictors usually have a 4-6 hour window before the onset of tissue necrosis.
Chemotherapeutic drugs have about 72 hours before necrosis begins.
How do you treat accidental intra-arterial injection of drugs?
Accidental intra-arterial injection of drugs can cause vasospasm and thrombosis.
Management includes injecting:
1. lidocaine
2. calcium channel blocker intra-arterially
3. A stellate ganglion block can also be useful.
Aspirin
Mechanism of Action?
Acetylsalicylic acid (ASA) blocks It is non-selective for COX-1 and COX-2 enzymes
Inhibition of COX-1 results in:
- the inhibition of platelet aggregation for about 7-10 days (average platelet lifespan)
- This prevents the production of pain-causing prostaglandins
- Stops the conversion of arachidonic acid to thromboxane A2 (TXA2), which is a potent inducer of platelet aggregation
Anti-platelet or Anti-coagulant?
Mechanism of Action
Abciximab (ReoPro)
Antiplatelet
Blocks GP IIb/IIIa receptors
Anti-platelet or Anti-coagulant?
Mechanism of Action
Cilostazol (Pletal)
Antiplatelet
Lowers calcium by elevating cAMP
Anti-platelet or Anti-coagulant?
Mechanism of Action
Clopidogrel (Plavix)
Anti-platelet
Inhibit ADP Receptor activation
Anti-platelet or Anti-coagulant?
Mechanism of Action
Dipyridamole
Anti-platelet
Lowers calcium by activating cAMP
Anti-platelet or Anti-coagulant?
Mechanism of Action
Eptifibatide (Integrilin)
Anti-platelet
Blocks GP2b3a receptors
Anti-platelet or Anti-coagulant?
Mechanism of Action
Prasugrel (Effient)
Antiplatelet
Inhibit ADP Receptor Activation
Anti-platelet or Anti-coagulant?
Mechanism of Action
Ticagrelor (Brilinta)
Anti-platelet
Inhibit ADP Receptor Activation
Anti-platelet or Anti-coagulant?
Mechanism of Action
Triofiban (Aggrastat)
Anti-platelet
GP2b3a receptor inhibitor
Anti-platelet or Anti-coagulant?
Mechanism of Action
Apixaban (Eliquis)
Anticoagulant
Direct Factor Xa Inhibition
Anti-platelet or Anti-coagulant?
Mechanism of Action
Dabigatran (Pradaxa)
Anti-coagulant
Direct Factor Xa Inhibition
Anti-platelet or Anti-coagulant?
Mechanism of Action
Fondaparinux (Arixtra)
Anticoagulant
Antithrombin mediated inhibition of factor Xa
Anti-platelet or Anti-coagulant?
Mechanism of Action
Low Molecular Weight Heparin
Anti-coagulant
Antithrombin mediated inhibition of serine proteinases
LMWH binds to anti-thrombin, a serine protease inhibitor, and creates a conformational change. This change accelerates its inhibition of activated factor X in conversion of prothrombin to thrombin. Thus,thrombin cannot convert fibrinogen to fibrin strands and clot formation.
Once this change occurs LMWH is freed and can bind to another anti-thrombin molecules. LMWH also directly inhibits thrombin as it is a heterogenous mixture of molecules, some containing enough polysaccharide sequence, but this effect is much less than that of unfractionated heparin.
Anti-platelet or Anti-coagulant?
Mechanism of Action
Rivaroxaban (Xarelto)
Anticoagulant
Direct factor Xa inhibition
Anti-platelet or Anti-coagulant?
Mechanism of Action
Unfractionated Heparin
Anticoaglant
Antithrombin mediated inhibition of serine proteinases
Anti-platelet or Anti-coagulant?
Mechanism of Action
Warfarin (Coumadin)
Anti-coagulant
Inhibit vitamin K dependent coagulation factors (II, VII, IX, X)
What ionic changes occur with NMDA receptor?
The NMDA receptor is an inotropic glutamate receptor that functions as a nonspecific ion channel when activated. Activation only occurs when glutamate is bound to the receptor AND the cell is depolarized. The receptor’s effects are primarily mediated via increased intracellular calcium.
How would magnesium effect Ketamine administration?
Administration of magnesium along with ketamine can potentiate the effects of ketamine.
What are the factors that will improve defibrillation?
1. Electrode Gel
2. Applying Force (if paddles)
- 11 lbs of pressure reduces resistance
3. Biphasic Defibrillation
- Uses less energy
4. Larger Electrodes (8-12 cm)
- Paddle size is inversely proportional to transthoracic resistance
- Smaller pads can cause myocardial necrosis
Compartment Syndrome?
6 signs/symptoms?
Diagnosis?
Treatment?
6 P’s
Pain out of proportion to the injury
Paresthesias
Pain on passive movement
Palpation of a tense hard compartment
Paralysis
Pulselessness (Late sign)
Diagnosis: Compartment Pressures >30 mmHg needs a fasciotomy within 6 hours
What is the equation for Arterial Oxygen Concent (CaO2)?
(Hgb * {4/3} * SaO2) + (0.003 * PaO2)
Modified Cormack-Lehane System
2b view shows what?
Only the posterior arytenoids and the epiglottis are visible
How does pulmonary mechanics change with low thoracic or lumbar epidurals?
Peak Expiratory Pressure will decrease the most
In healthy patients, lumbar or low thoracic epidural anesthesia produces small (≤ 10%) changes in most pulmonary function parameters. However, PEP and cough strength are significantly reduced (10-40% and up to 50%, respectively) from baseline.
These two parameters are more dependent on abdominal musculature which has a higher degree of motor blockade than thoracic musculature from a lumbar or low thoracic epidural.
Note a disproportionately greater decrease in peak expiratory pressure with only a small decrease in FEV1 is in line with FEV1 being relatively effort-independent (lower peak expiratory pressure in a weaker patient is conceptually equivalent to lower effort).
How does tidal volume change with thoracic and lumbar epidurals?
Tidal volume is typically unchanged, even with a high thoracic epidural.
Definte and quantitatively define Different Levels of MAC
MAC-Awake?
MAC?
MAC-BAR?
MAC-Awake
The MAC-awake is the MAC value at which voluntary reflexes (e.g., a patient will no longer open his or her eyes to command, shouting, or shaking) and perceptive awareness are lost. It varies between 15-50% of standard MAC
MAC
minimum alveolar concentration of an inhaled anesthetic at one atmosphere that prevents movement in response to a surgical stimulus (specifically, an abdominal incision) in 50% of patients.
MAC-BAR
MAC value at which the adrenergic response (e.g., hemodynamic, sudomotor) to noxious stimuli is blunted. This has been found to be approximately 50% higher than standard MAC. Some studies estimate this value as 1.7-2.0 MAC.
MAC-awake in generally higher on induction or emergence?
Why is this?
Interestingly, the MAC-awake is generally higher at induction (40-50% standard MAC in order for loss of consciousness) than at emergence (as low as 15% standard MAC to regain consciousness)
Although the mechanism is not fully understood, it is likely due to the differences in alveolar wash-in and wash-out of anesthetic gases.
The theory of neural inertia is another proposed mechanism.
Neural inertia is defined as the tendency of the central nervous system to resist transitions between arousal states
Loss of awareness and recall typically occurs at what MAC?
Which volatile anesthetic has the most recall blocking activity and which has the least?
The loss of awareness and recall typically occurs at 0.4-0.5 standard MAC
Isoflurane likely has the most recall-blocking activity and nitrous oxide the least
Current ASA guidelines recommend maintaining at least what MAC during general anesthesia to significantly reduce the risk of awareness?
A MAC at this level effectively reduces awareness to what ratio of patients for recall of verbal stimulus.
Current ASA guidelines recommend maintaining at least 0.7 MAC during general anesthesia to significantly reduce the risk of awareness.
A MAC value of 0.627 effectively reduces awareness to 1 in 100,000 for recall of verbal stimulus.
If you have a patient with suspected TBI with GCS of 3, what is the goal of cerebral perfusion pressure (CPP)?
Even a single episode of hypotension decreases cerebral perfusion enough to affect outcomes. The CPP value to target lies within the range of 50-70 mm Hg according to current BTF (Brain Trauma Foundation) guidelines.
Avoid CPP < 50 mmHg
As far as the upper limit, aggressive attempts to maintain CPP > 70 mmHg with fluids and pressors should be avoided because of the risk of acute respiratory distress syndrome (ARDS). However, if a patient with intact autoregulation is maintaining a CPP > 70 mmHg spontaneously, that is acceptable.
How do you classify mild, moderate, and severe TBI?
TBIs are categorized by level of severity: mild, moderate, and severe.
A mild TBI is associated with a Glasgow Coma Scale (GCS) score of 13-15 and minimal to no loss of consciousness.
A moderate TBI is associated with a GCS of 9-12 and a loss of consciousness of 30 minutes or more.
A severe TBI is associated with a GCS of 3-8.
When should you start treating increased ICP in TBI?
Treatment should be initiated with ICP thresholds above 20 mmHg.
How do you adjust methadone dosing with ESRD?
Methadone also needs little adjustment in patients with renal disease.
Why should you be cautious with ESRD patients when considering giving hydromorphone?
Buildup of renally-excreted hydromorphone-3-glucuronide can lead to neuroexcitatory effects including agitation, restlessness, and myoclonus (neuroexcitatory & pro-convulsant properties)
What are two aspects of Ketorolac side effects that you should consider before administering?
Prevents the synthesis of prostaglandins.
Inhibition of PG synthesis can affect renal glomerular blood flow by causing vasoconstriction of the afferent arterioles of the glomeruli
(KIDNEY ISSUES)
Reversibly inhibits the cyclooxygenase (COX) enzyme which prevents the formation of thromboxane from arachidonic acid.
Thromboxane promotes platelet aggregation and its absence can lead to increased bleeding.
(BLEEDING AND THROMBOSIS ISSUES)
Besides Lead II, What is the best lead to monitor atrial dysrhythmias?
Lead V1 is the second-best lead for monitoring atrial arrhythmia and when using a full twelve lead ECG considering these two leads together is most effective.
The normal p-wave morphology in V1 is biphasic with an initial upward deflection as depolarization travels from the SA node anteriorly through the right atrium. There is then a terminal deflection as the current passes posteriorly through the left atrium.

What are the ways you can prevent contrast induced nephropathy in patients?
#1 = Fluid Hydration
Antioxidants such as N-acetylcysteine and ascorbic acid may also play a role in renal protection but are not as effective as fluid hydration alone.
What is the equation of SVR?
What are the normal values?
SVR = [80 * (MAP – RAP)] ÷ CO
MAP = mean arterial pressure (mm Hg)
RAP = right atrial pressure (mm Hg), central venous pressure is commonly substituted for RAP
CO = cardiac output (L/min)
80 = conversion factor which changes mm Hg/L/min (Woods unit) to dynes * sec/cm^5
What is the equation for PVR?
What are the normal values?
PVR = [80 * (MPAP – PAOP)] ÷ CO
- *MPAP** = mean pulmonary arterial pressure (mm Hg)
- *PAOP** = pulmonary artery occlusion pressure or pulmonary capillary wedge pressure (mm Hg)
- *CO** = cardiac output (L/min)
- *80** = conversion factor which changes mm Hg/L/min (Woods unit) to dynes * sec/cm^5
Normal Value = 20–130
How do you convert SVR or PVR into woods units?
Conversion of dynes * sec/cm^5 to Woods units may be achieved by dividing SVR by 80.
For example, 1520 / 80 = 19 Woods units
What are some of the complications of brachial arterial lines?
1. Thrombosis (Vascular > Infection)
2. Bleeding
3. Pseudoaneurysms
4. Arterial-Venous Fistula
5. Infection (Lower Risk)
6. Median Nerve Injury
There have been concerns that brachial artery catheterization can lead to limb ischemia due to lack of collateral vessels; however, this has not been shown to be true.
The rare instances of ischemia from brachial artery use were resolved with the catheter removal.
The radial artery is even more unlikely to have ischemia because of multiple collateral vessels.
What is the ED95 of neuromuscular blockers mean?
ED95 is the effective dose of a neuromuscular blocking drug required to achieve 95% block of a single twitch in 50% of individuals who receive this drug and dose.
What is the ED95 and Intubating dose of:
Cisatracurium?
Pancuronium?
Vecuronium?
Rocuronium?
NDNMB ED95 (mg/kg)
Dose For Intubation (mg/kg)
Cisatracurium
- 04 (0.032 - 0.05)
- 15-0.2 (3.75 - 5 x ED95)
Pancuronium
- 07
- 08-0.12 (1.2 - 1.8 x ED95)
Rocuronium
- 3
- 6-1.0 (2 - 3.3 x ED95)
Vecuronium
- 05
- 1-0.2 (2.3 - 4.7 x ED95)
What is the P50 value?
What is the normal value for an adult?
Draw the dissociation curve with axis labeled
The term P50 is designated as the partial pressure of oxygen (in mm Hg) when oxygen saturation (SaO2) is 50%.
In a normal adult, P50 is 27 mm Hg.

How does a newborn shift on the hemoglobin dissociation curve?
What is the P50 value?
Why is that?
Left Shift
In newborns, oxygen affinity is very high and P50 is very low (approximately 18 mm Hg).
Due to:
- Hemoglobin F, which is produced in-utero, and is useful for oxygen transfer from the maternal blood to the fetus. The presence of hemoglobin F
- Low 2,3-diphosphoglycerate (2,3-DPG) levels in newborns result in a very low P50 (Note, this would cause a left shift as well)

How does the hemoglobin dissociation curve change through infancy?
What is the P50 value?
Why is this?
Right Shift
After birth, levels of hemoglobin F begin to decline (Right shift) resulting in a decrease in the total hemoglobin concentration. This results in a physiological anemia of infancy. During this same period of time, 2,3-DPG levels begin to increase causing the oxyhemoglobin dissociation curve to shift to the right (P50 increases). The P50 will surpass that of an adult (27 mm Hg) and will reach a maximum of 30 mm Hg by about 12 months of age
- Children will continue to have a higher P50 for the first decade of life.

What are the major factors that contribute to Right and Left Shift is the Hemoglobin dissociation curve?
P50 increases due to: acidosis, hypercarbia, hyperthermia, increased 2,3-DPG. Shift “RIGHT”:
Rise In 2,3-DPG, H+, and Temp.
P50 decreases due to: alkalosis, hypocarbia, hypothermia, decreased 2,3-DPG.
When does P50 value reach normal adult level?
Age 10
What is the Haldane Effect effect illustrate?
Draw the respective curve.
Illustrates that the oxygenation of hemoglobin lowers the affinity of hemoglobin for carbon dioxide
The Haldane effect describes the relationship between the carbon dioxide dissociation curve in blood and oxyhemoglobin (HbO2).
The CO2 dissociation curve, specifically, shifts to the right when the concentration of HbO2 increases in the blood.
This curve is linear since the CO2 content and the partial pressure of CO2 (PCO2) are directly correlated in a given sample of blood. The curve may be interpreted such that an increase in HbO2 results in an increased dissociation of CO2 from Hb (i.e., an increase in PCO2 but an overall unchanged value for CO2 content).
Conversely, if the concentration of HbO2 is reduced, then the affinity for carbon dioxide increases and less CO2 is available for gas exchange.

What is the Bohr Effect?
Draw the respective curve
The Bohr effect describes the binding effect of H+ to Hb chains and the oxygen release and dissociation that occurs thereafter.
Acidosis, for example, will shift the oxygen-hemoglobin dissociation curve toward the right, resulting in less attraction between Hb and O2.
Ultimately, this will permit more CO2 to be transported from the tissue to the lungs during gas exchange. The curve will shift to the left for a low PCO2 and high pH.

Supratherapeutic INR
Mild (INR < 5) & No Bleeding OR No need for emergent surgery
Treatment?
Treatment: Withholding warfarin for several days is recommended.
The half-life of warfarin therapy is 2 to 4 days thus the effects may not be seen for several days.
Supratherapeutic INR
Moderate INR (between 5 and 9 No Bleeding OR No need for emergent surgery
Treatment?
Treatment: Vitamin K can be administered orally or parenterally
- Typical adult dose is 1-2mg)
- There is no difference in efficacy between oral and intravenous vitamin K
- Higher risk of anaphylaxis with parenteral formulations; thus oral is preferred if the patient can tolerate and absorb the medication
Supratherapeutic INR
Severe INR (> 9) or there is life threatening bleeding
Treatment?
Treatment: Higher doses of vitamin K may be attempted (between 5-10mg).
Higher doses of vitamin K may render the patient temporarily resistant to further warfarin therapy for days to weeks.
Supratherapeutic INR
Emergent Surgery or If significant bleeding is present
Treatment?
1. Prothrombin complex concentrates
2. IV Vitamin K
What are the two formulations of PCC?
4-factor concentrates are preferred as they contain all vitamin K dependent coagulation factors (II, VII, IX, and X) (1972)
3-factor concentrates contain factors II, IX, and X and should be supplemented with recombinant factor VIIa to completely reverse anticoagulation.
What are the contraindications to PCC therapy?
Contraindications:
1. DIC
2. HIT - PCCs are human plasma derived products that contain low doses of heparin to prevent clotting factor activation.
What are the major concerns with FPP administration?
There are certainly risks associated with FFP transfusion; most notable are:
Transfusion related acute lung injury (TRALI)
Febrile reactions
Allergic reactions
Transfusion associated circulatory overload (TACO).
What does ASA deem the 4 distinct depths of anesthesia?
Minimal Sedation
Moderate Sedation
Deep Sedation
General Anesthesia
What are the criteria for the 4 depths of anesthesia
(Draw the table)
- Responsiveness
- Airway reflexes
- Ventilation
- Cardiovascular Function
- *Minimal Sedation**
- Normal response to verbal stimulation
- Airway reflexes, spontaneous ventilation, and cardiovascular function are all unaffected
- *Moderate Sedation**
- Purposeful response to verbal or tactile stimulation
- Spontaneous ventilation is adequate and no airway intervention is required
- Cardiovascular function is usually maintained
- *Deep Sedation**
- Purposeful response to repeated or painful stimulation (reflex withdrawal from a painful stimulus is NOT considered purposeful response)
- Spontaneous ventilation may be inadequate and airway intervention may be required
- Cardiovascular function is usually maintained
- *General Anesthesia**
- Unable to arouse even with painful stimulus
- Spontaneous ventilation is frequently inadequate and airway intervention is often required
- Cardiovascular function may be impaired

How does the Frank Starling Curve change for Heart Failure Treatments?
Label the Axes
Include:
- Ionotropes
- Vasodilators
- Diuretics
- Ionotropes and Vasodilators
- Ionotropes, Vasodilators and Diuretics

A = Diuretics
B = Ionotropes + Vasodilators + Diuretics
C = Vasodilators
D = Ionotropes and Vasodilators
E = Ionotropes only

What is the elimination of neostigmine?
50% renal excretion
Eliminiation Half life of neostigmine:
Without renal failure/ESRD?
With renal failure/ESRD?
Without = 77 minutes
With = 181 minutes
Duration of Action of Rocuronium and Vecuronium?
46 - 73 minutes
Rocuronium
Metabolism?
Elimination?
Metabolism = 70% Hepatic
Elimination = 10% Renal Elimination
Vecuronium
Metabolism?
Elimination?
Metabolism - 50-60% Hepatic
Elimination - 25% Renal
What triggers the sub-ambient pressure alarm on the ventilator?
What are some examples:
Triggered when the pressure in the breathing circuit falls below atmospheric pressure by a predetermined amount (typically below -10 cm H2O).
Examples
- Inhalation against an increased resistance in the circuit
- Inhalation against a collapsed reservoir bag
- A malfunctioning active closed scavenging system (excessive vacuum or valve dysfunction)
- A blocked inspiratory limb during exhalation
- Accidental Placement of Nasogastric Tube or Endoscope within the trachea and use of suction = Loss of Tidal Volume and Negative pressure
What is myoclonus?
What is thought to derive from?
What is seen on EEG?
Myoclonus describes sudden jerking movements, such as those sometimes observed during induction of anesthesia
It likely results from inhibition of descending cortical inhibitory pathways.
Myoclonus can resemble seizures but EEG evidence of seizures is lacking during these episodes.
Methohexital has what side effects?
1. Myoclonus
2. Pain on Injection
3. Hiccups
What is the mechanism of action of Fenoldopam?
Dopamine-1 agonist = Increases renal blood flow despite decreased systemic arterial blood pressure
Natriuretic - Sodium excretion
Diuretic - Free water excretion
Direct renal vasodilator and can produce hypotension
What are some indications for Fenoldopam?
- A dopamine D1 receptor agonist that is used as an antihypertensive agent
- It lowers blood pressure through arteriolar vasodilation.
- Used as renal protector when renal vasoconstriction is anticipated
- Hypertensive Crisis in those with decreased renal function
How does phenytoin affect neuromuscular blockade?
Acute phenytoin administration potentiates the neuromuscular blockade of aminosteroid NDNBDs.
Chronic phenytoin administration increases a patient’s resistance to the effects of NDNBDs and reduces their duration of action by as much as 50%
For benzylisoquinoloine NMB = Variable
Why (Mechanism) are Non-depolarizing neuromuscular drugs affected by Phenytoin?
1) Increased metabolism via cytochrome P450 enzymes induction (this may explain why there is a clear effect with the aminosteroid NDNBDs, which rely on hepatic metabolism, but not with the benzylisoquinolines which undergo hepatic-independent Hofmann elimination and ester hydrolysis)
2) Increased postjunctional acetylcholine receptor density (the weak neuromuscular blocking properties of phenytoin, see below, results in postjunctional acetylcholine receptor upregulation)
3) Decreased sensitivity at the receptor sites
4) Increased end-plate anticholinesterase activity
Duing a surgery with general anesthesia, what aspect of the surgery requires the highest concentration of MAC to prevent movement:
DL, ETT placement, Surgical incision, or Tetanus to nerve stimulation?
ETT placement (MOST)
Surgical INcision
DL
Tetanus by nerve stimulation (LEAST)
Butorphanol
Mechanism?
Partial Opioid Agonist and Mixed Agonist-Antagonist
What is a normal CVP?
0 - 8 mmHg
What is a normal Mean Pulmonary Artery Pressure?
10 - 18 mmHg
What is a normal PCWP (Pulmonary Capillary Wedge Pressure)?
25 mmhg
What is a normal Cardiac Index?
2.5 - 4.2
How would PEEP improve hemodynamics in a patient with acute on chronic systolic heart failure?
Changes in:
Intrathoracic Pressure
RV Afterload
Preload
CVP
PAP
CI
PCWP
- *Raises** Intrathoracic Pressure
- *Increase** in RV Afterload
Decrease in Preload
Increase in CVP
Increase in PAP
Improved CI
Improved PCWP
What are the criteria of the Aldrete Scoring System used to dischage patients from Phase I of the PACU?
Consciousness
Activity (Moving extremities on command)
Respiration
Oxygen Saturation with O2 requirements
Circulation (BP compared to preop)
What type of shunt is created during one lung ventilation?
Right to Left Shunt
What is the most common and reliable sign of Cyanide Toxicity?
Why is this?
Elevated Anion Gap Metabolic Acidosis
Cyanide primarily causes toxicity by impairing cellular aerobic respiration. The cyanide ion (CN-) binds to the ferric ion (Fe3+) in mitochondrial cytochrome-c oxidase, inhibiting the final stage of the electron transport chain. Depletion of cellular ATP and the lactic acid produced by anaerobic metabolism can lead to profound acidosis.
Cyanide Toxicity
- Signs and Symptoms?
- PaO2 levels?
- SvO2 levels?
Signs/Symptoms:
Altered mental status, weakness, headaches, loss of consciousness, seizures, respiratory failure, and cardiac arrest, The patients blood sample may appear “cherry red” due to normal circulating levels of oxygen with impaired utilization.
PaO2 increases (Oxygen present but cannot be utilized)
SvO2 increased (oxygen present but not being utilized)
Mapleson Circuit Fresh Gas Flow (FGF) Requirements:
- FGF Mapleson A requirements for spontaneous ventilation?
- FGF Mapleson D, E, or F requirements for spontaneous ventilation?
- FGF Mapleson D, E, or F requirements for controlled ventilation?
A = Minute Ventilation
B = 2-3 times minute ventilation
C = 1-2 times minute ventilation
When a patient is on chronic dantrolene therapy, what needs to be continously monitored?
LFT’s
- Liver Dysfunction and potentially liver failure is possible
How does distance to radiation exposure protect you?
Ex: If you double the distance from the C-arm in the room, how does that decrease your radiation exposure?
Radiation intensity (exposure) with respect to distance decreases according to the inverse square law: I ∝ 1 / r^2.
Accordingly, doubling the distance from a radiation source decreases exposure by a factor of 4.
How does Bicarb administration affect BP and Calcium Levels?
Hypotension
Hypocalcemia (Transiently binds calcium and should be cautious in hypocalcemic patients)
To what degree (quantitatively) does 50 mEq of Bicarbonarte affect PaCO2?
Hypercarbia - The reaction of 50 mEq bicarbonate produces approximately 1,250 mL of CO2 which must be exhaled and is seen as a transient increase in EtCO2.
How does bicarbonate affect pulmonary vasculature?
Redistribution of Blood to the Pulmonary Vasculature
What nerves are required to perform an awake fiberoptic intubation?
Simply, CN 9 and CN 10
Specifically:
1. Internal Branch of Superior Laryngeal Nerve of CN 10
- CN 9 - Glossopharyngeal Nerve
- Recurrent Laryngeal Nerve of CN 10

Why do spinals result in hypotension?
- Arteriodilation - Due to sympathectomy
Reduction in afterload
2. Venodilation - Due to sympathectomy
Venodilation causes a reduction in preload and tends to have a more dramatic effect than arterial dilation because 75% of the total blood volume is in the venous system.
- Bradycardia - 10-15% from the spinal
- Blockade of the cardiac accelerator fibers can prevent an increased heart rate in response to hypotension, which would lead to further hypotension and bradycardia.
What is the concept of the Bezold-Jarisch Reflex?
Parasympathetic-mediated reflex occurs when stretch receptors located mainly in LV respond to an acute decrease in LV preload
The result is bradycardia and reduced contractility (and resultant hypotension).
This reflex is thought to occur to allow the ventricle additional time to fill and increase preload.
For a spinal, what dermatomes are typically affected above the spinal?
2-6 Dermatomes sensory block above level of the sensory block
What is Fractional Area Change?
Quantitative echocardiographic technique used to determine left ventricular ejection fraction.

Aortic Stenosis
What are the 3 metrics looked at to determine aortic stenosis gradients?
- Jet Velocity - (3-4 m/s)
- Mean Gradient - (25 - 40 m/s)
- Valve Area (1.0 - 1.5)

What degree angle would affect Velocity measurement as assessed by Doppler ultrasound is governed by the Doppler equation?
>20 degrees
Volatile Anesthetic effects on CBF and CMRO2 at:
>1 MAC
<1 MAC
≥1 MAC increase CBF and decrease CMRO2, causing an uncoupling of the flow-metabolism relationship.
<1 MAC = CBF balances and remains the same
How do Barbituates affect:
Cerebral Flow Metabolism & Cerebral Autoregulation?
CBF and CMRO2 % change in induction?
CBF and CMRO2 % change of isolectric EEG?
The cerebral flow-metabolism relationship and cerebral autoregulation remain intact with the use of thiopental.
Thiopental decreases both CBF and CMRO2 by 30% with induction doses
and by 50% upon achievement of an isoelectric EEG.
When would burst suppression on EEG be the goal target of reducing CMRO2?
What does burst suppression indicate?
Burst suppression on EEG is the goal target of reducing CMRO2 during an open cerebral aneurysm clipping.
Burst suppression sufficiently indicates depressed CMRO2 while providing predictability of recovery and awakening once the IV anesthetic is turned off.
What are the classic signs of Sodium Nitroprusside Toxicity?
- Elevated Mixed Venous Oxygen (PvO2)
Since cells cannot use oxygen and aerobic respiration, PVO2 becomes elevated.
- SNP (Sodium Nitroprusside) Tachyphylaxis
- Metabolic Acidosis (Lactate Buildup)
Amyl NItrate is an antidote to what poisoning?
How does this work?
Amyl nitrate works as an antidote for cyanide poisoning by converting Hb to MetHb which avidly binds cyanide, converting it to the nontoxic cyanomethemoglobin.
What are the 3 treatments for cyanide poisoning?
- Hydroxycobalamine
- Amyl NItrate
- Sodium Thiosulfate

What is the onset and duration of action of:
Cimetidine
Ranitidine
Famotidine
Cimetidine 1-1.5 hours, 3-4 hours
Ranitidine 1 hour, 9-10 hours
Famotidine 1 hour, 10-12 hours
What is the mechanism of action of Histamine H2 receptor antagonists?
Histamine H2 receptor antagonists (e.g. cimetidine, ranitidine, famotidine, nizatidine) block histamine from inducing acidic gastric fluid secretion by parietal cells.
This effectively raises gastric pH.
Raising the gastric pH prior to induction of anesthesia can reduce the severity and risk of perioperative aspiration pneumonitis.

Why do Barium Hydroxide absorbents produce more CO than soda lime?
Barium hydroxide absorbent preparations produce more carbon monoxide than soda lime due to the decreased water content of barium hydroxide absorbents.
Between different CO2 absorbers, what is the greatest at CO2 absorption?
Soda Lime
Calcium Hydroxide
Barium Hydroxide
Soda Lime
What is the composition of Soda Lime?
80% calcium hydroxide
15% water
4% sodium hydroxide
Therefore, higher-moisture absorbents (e.g., soda lime) have more water content available to react with CO2 leading to an increased CO2 absorptive capacity.
What are the factors that will increase CO production?
Desiccated CO2 absorbents
Increased Temperature
Low Fresh Gas Flows
What volatile anesthetic produces the most heat?
What voltaile anesthetic produces the most Carbon Monoxide?
CO2 absorbents + Sevoflurane = Most Heat
CO2 absorbents + Desflurane = Most CO
What absorber is the most likely to produce compound A with sevoflurane?
Soda Lime
Calcium Hydroxide
Barium Hydroxide
Barium Hydroxide
*These have been removed from US markets
Why do Calcium Hydroxide absorbents offer less CO2 absorption?
Lack Strong Bases (NaOH and KOH)
The maximum CO2 absorbed by calcium hydroxide is 10.2 liters per 100 grams absorbent whereas soda lime can absorb 26 liters of CO2 per 100 grams absorbent.
Which Absorber has the lowest incidence of compound A and Fire Production?
Soda Lime
Calcium Hydroxide
Barium Hydroxide
Calcium hydroxide absorbents, due to lower reactivity, have the lowest incidence of compound A and fire production
If you suspect a colleague of having a substance abuse problem, what is the correct answer for the exam in terms of what to do?
If you suspect a colleague of having a substance abuse problem, you should consult your state physicians health program before intervention or confrontation.
What are the indications for FFP?
- Correction of excessive microvascular bleeding (PT >1.5 times normal, PTT >2 times normal, or INR >2)
- Correction of coagulation factor deficiencies if the patient has been transfused with more than one blood volume (approximately 70 ml/kg)
- Correction of coagulation factor deficiencies for which there are no specific replacements
- Heparin resistance (antithrombin III deficiency) in a patient requiring heparin
- Coagulopathy related to hepatic insufficiency
- TTP
- Reversal of Warfarin (PCC another option)
What is the reason that FFP is used to treat Thrombotic thrombocytopenic purpura (TTP)?
Thrombotic thrombocytopenic purpura (TTP) is a platelet destruction disorder.
The inherited type involves a deficiency of vWF-cleaving protease activity (ADAMTS13 deficiency).
Fresh frozen plasma is administered in order to replete the ADAMTS13 enzyme.
Plasmapheresis may be used to treat the acquired type of TTP as it removes the acquired antibodies that damage the ADAMTS13 enzyme.
Esmolol
Selectivity?
Half Life?
Metabolism?
Esmolol is a β1-selective short acting (t1/2 = 9 min) agent that is
Rapidly metabolized by:
- Red blood cell (RBC) esterase
- Minimally hydrolyzed by pseudocholinesterase.
How do Beta Blockers affect the heart?
Beta-blockers reduce:
1. blood pressure
2. myocardial oxygen consumption
primarily by
1. decreasing heart rate
2. contractility.
They also have anti-arrhythmic properties by decreasing AV conduction and automaticity, and by increasing the atrial refractory period.
Propranolol selectivity?
Propranolol is non-selective and inhibits β1 and β2 receptors
Labetolol Selectivity?
β1, β2, and α-1 receptor blocker.
What is the ratio of selectivity of IV vs. PO Labetolol?
What is the unique about the way that labetolol can function as an anti-hypertensive?
The ratio of relative α:β potency of IV labetalol is approximately 1:7 whereas PO labetalol is 1:3.
The nature of this drug can be seen intraoperatively when labetalol administration results in decreased blood pressure without baroreceptor-triggered reflex tachycardia (as can be seen with pure vasodilating agents)
Besides labetolol, what is the only other beta blocker that has alpha antagonism properties?
Carvedilol
How are beta blockers (outside of esmolol) metabolized?
Hepatic Clearance
Exception: Atenolol (Which is renally cleared)
What is the pathophysiology of Propofol Infusion Syndrome?
The pathophysiology of PRIS relates to propofol’s ability to impair cellular free fatty acid utilization and mitochondrial activity leading to inadequate aerobic metabolism and increased reliance on anaerobic metabolism.
What arer some of the downstream effects of Propofol Infusion Syndrome?
Cardiac and skeletal muscle are particularly susceptible leading to muscle damage or necrosis that can cause cardiac failure and rhabdomyolysis.
lactic acidosis
hyperkalemia
renal failure
Hypertriglyceridemia
Hepatomegaly
Pancreatitis secondary to HLD
What color is urine with propofol infusion syndrome?
Green
Phenol excretion can cause this
How does respiratory alkalosis affect Calcium, Potassium and Phosphate levels?
All drop
What is the incidence of infection of central line?
15%
What happens if you rotate the head to the right >30 degrees to place a LEFT IJV Central Line?
It has been shown that with head rotation greater than 30 degrees that the left carotid artery and jugular vein overlap more than on the right.
If doing a carotid massage, what side should you do it on?
1. Carotid embolization on the left poses a greater risk as the left cerebral hemisphere is dominant in the majority of the population. This is also one of the reasons why right-sided carotid massage is preferred over left-sided massage.
- Another reason is that some investigations have found a greater cardioinhibitory effect on the right side.
What is the pathophysiology of performing a carotid massage?
Exert Pressure on Carotid Sinus and baroreceptors at the Transverse process of C6

The AFFERENT nerve impulses of carotid sinus baroreceptors are transmitted by the Hering’s nerves to the glossopharyngeal nerve (CN IX) (see image below).
Arterial wall stretch at the carotid sinus, within the internal carotid artery, activates the afferent impulse.
This activation leads to stimulation of the nucleus tractus solitarius (NTS) in the caudal medulla.
The NTS sends excitatory signals to other regions of the medulla which in turn inhibit stimulation of the preganglionic intermediolateral nucleus of the spinal cord which mediates the body’s sympathetic innervation.
The NTS may also stimulate vagal nuclei to activate the parasympathetic nervous system via the vagus nerve. These two effects then lead to bradycardia and hypotension.
What is the a wave on CVP tracing responsible for?
a wave: atrial contraction
What is the C wave on CVP tracing?
c wave: TV bulging into RA during RV isovolumetric contraction
What is the x descent on CVP tracing?
x descent: TV descends into RV with ventricular ejection and atrial relaxation
What is the v wave on CVP tracing?
v wave: venous return to and systolic filling of the RA
What is the y descent on cvp waveform?
y descent: atrial emptying into RV through open TV
How does atrial fibrillation reveal itself in CVP waveform?
absent a wave
How does Tricuspid Regurgitation reveal itself on CVP waveform?
Tall c waves
Tall v waves
Loss of x descent
How does Tricuspid Stenosis reveal itself on CVP wavefrom?
Tall a
Tall v waves
Minimal y descent
How does RV Ischemia present on CVP tracing?
Tall a & v waves
Steep x & y descent
M or W configuration
How does Pericardial Constriction present on CVP tracing?
Tall a & v waves
Steep x & y descent
M or W configuration
How does Cardiac Tamponade present on CVP tracing?
Dominant x descent
Minimal y descent
Draw a CVP overlapping with EKG.

What is the treatment for Methemoglobinemia resulting from G6PD?
Ascorbic Acid
Ascorbic acid also known as vitamin C, functions as an electron receptor and aids in the reduction of Fe3+ to Fe2+.
Ascorbic acid functions to reduce hemoglobin slower than methylene blue does. However, ascorbic acid does not have the hemolytic effect that methylene blue does in a G6PD deficient patient.
In a patient with G6PD deficiency the hexose phosphate pathway is dysfunctional and free radicals develop, which causes red blood cell lysis.
What is the treatment of Methemoglobinemia?
Methylene blue is often the drug of choice when treating methemoglobinemia.
Methylene blue provides an electron receptor for the reduction of methemoglobin using NADPH produced from the hexose phosphate pathway.
In a patient with G6PD deficiency the hexose phosphate pathway is dysfunctional and free radicals develop, which causes red blood cell lysis. Therefore, methylene blue should be avoided in patients with G6PD deficiency. Additionally, methylene blue is a potent reversible monoamine oxidase inhibitor (MAOI) and may interact with serotonergic psychiatric medications leading to life-threatening serotonin syndrome.
Other than G6PD deficiency, when should you avoid giving methylene blue?
Additionally, methylene blue is a potent reversible monoamine oxidase inhibitor (MAOI) and may interact with serotonergic psychiatric medications leading to life-threatening serotonin syndrome.
If an IV cannot be obtained in a patient suffering from cyanide toxicity, what is the treatment of choice?
Amyl nitrite is used to treat cyanide toxicity.
Amyl nitrate oxidizes Fe2+ to Fe3+.
Cyanide binds to methemoglobin more actively than it binds to normal hemoglobin.
Thus, by inducing a low-level of methemoglobinemia the methemoglobin in turn can sequester cyanide as cyanmethemoglobin.
Methemoglobinemia:
What occurs to Iron?
What type of anemia is caused?
Left or Right shift on Oxygen Dissociation curve?
Absorbance?
SpO2?
What should be used to monitor?
Methemoglobinemia occurs when hemoglobin becomes oxidized from Fe2+ to Fe3+.
Methemoglobin is unable to bind oxygen and causes a functional anemia, in addition to left shifting the oxygen dissociation curve.
Methemoglobin has an absorbance of 630 nm, which correlates to an oxygen saturation of 84-86% on pulse oximetry.
Co-oximetry should be used to aid in the diagnosis and treatment of methemoglobinemia. If unavailable, blood can be sent for a methemoglobin level.
Why is it that Carbon Monoxide Poisoning has a SpO2 of ~100% at high readings?
The COHb is interpreted by the pulse oximeter as a mixture of approximately 90% HbO2 and 10% HHb.
Thus, at high levels of COHb, the pulse oximeter will overestimate true SaO2, as may occur in patients with recent CO exposure.
How is SpO2 affected with Methemoglobinemia?
MetHb is formed when the heme iron is oxidized from the ferrous (Fe2+) to the ferric (Fe3+) state.
metHb is very dark and tends to absorb equal amounts of red and infrared light, resulting in a red:infrared ratio of 1.
When extrapolated on the calibration curve, a ratio of 1 corresponds with a saturation of 85%.
Thus, as metHb increases, SpO2 approaches 85% regardless of the true level of HbO2.
In order for Methemoglobinemia to be diagnosed, how much is the % increase?
What 4 drugs do anesthesiologist give that can cause this?
Drugs capable of causing methemoglobinemia (defined as greater than 1% metHb) include nitrates, nitrites, chlorates, nitrobenzenes, antimalarial agents, amyl nitrate, nitroglycerin, sodium nitroprusside, and local anesthetic agents.
What 3 dyes in the OR can produce SpO2 decreases?
How long do these decreases last?
Injections of methylene blue produce a large and consistent spurious decrease in SpO2, with readings remaining below baseline for 1 to 2 minutes.
Injection of indocyanine green decreases SpO2 to approxi- mately 80% to 90%, which lasts for a minute or less.
Injection of indigo carmine decreases the SpO2 the least, with the decrease lasting approximately 30 seconds.
What is the pathophysiology of Botulism?
(Include organism, mechanism of the bacteria in molecular detail)
Botulism is caused by the anaerobic bacillus, Clostridium botulinum.
Flaccid neuroparalysis occurs due to the prevention of acetylcholine (ACh) release from vesicles at the NMJ.
Acetylcholine is carried to the nerve terminal by vesicles which then fuse with the nerve terminal membrane via soluble N-ethylmaleimide-sensitive fusion associated protein receptor (SNARE) proteins. The collection of SNARE proteins facilitate exocytosis and then “repackaging” or reuptake of ACh.
The various toxins of C. botulinum are zinc endopeptidases that cleave the SNARE proteins and stop the fusion and release of ACh into the nerve terminal, as well as prevent reuptake back into the terminal.

What is the mechanism of action of Tetanus?
Include the organism and also the mechanism of action in molecular detail
The tetanus toxin from Clostridium tetani travels via neuronal retrograde transport up the motor neuron and enters the presynaptic terminal of inhibitory interneurons within the spinal cord. Normal modulation of fine motor movement from descending motor pathways triggers the interneurons to exert their inhibitory effects via γ-aminobutyric acid (GABA) release.
- The toxin prevents the release of GABA from the interneurons. Therefore, the inhibitory mechanism is inhibited and spastic neuroparalysis occurs.

What are the 4 sites for IO Placement?
1. Sternum
2. Proximal Humerus
3. Proximal Tibia
4. Distal Tibia

For the 4 sites of IO placement, what is the drainage of each IO site?
- Sternum = Azygos and Internal Mammary Vein
- Proximal Humerus = Axillary Vein
- Proximal Tibia = Popliteal Vein
- Distal Tibia = Greater Saphenous Vein
What are the advantages of humeral and sternal IO?
Humeral = Flow rates are higher than tibial (200 vs. 100 mL/min)
Sternal = Fast uptake of drugs (80-110 seconds) which is not that much longer than central access (60-80 seconds)
Explain in terms of ionization, pKa why Alfentanil has such a high onset of action?
pKa = 6.5
Highly un-ionized form (90%)
Lower Lipid Solubility

Compare Alfentanil to Fentanyl in terms of:
Onset:
Duration:
Potency:
Remembering “4” will get you in the appropriate range for differences between alfentanil and fentanyl.
Alfentanil has about 4 times faster onset.
Alfentanil lasts about 1/4 the duration.
Alfentanil is about 1/4 the potency (4x the dose of fentanyl).
Desflurane effects on:
BP?
Afterload?
HR?
Myocardial Function?
CO?
LV Diastolic Function?
Desflurane primarily decreases arterial pressure by decreasing afterload.
Desflurane increases heart rate, particularly when the concentration is quickly increased
Also causes dose-dependent depression of myocardial function.
Cardiac output is maintained and there is no significant effect on left ventricular diastolic function.
What are some of the extrapyramidal side effects to watch for?
- Acute dystonias (abnormal movement or posturing due to involuntary/sustained muscle contractions)
- Akathisia (restlessness and need to be in constant motion)
- Tardive dyskinesia (involuntary repetitive or purposeless movements).

What is the dose of metoclopramide that is needed to prevent PONV?
What is the respective risk of Extrapyramidal effects with these doses?
Metoclopramide has weak antiemetic effect at 10 mg (< 20 mg) and recommended dosing for PONV is 25-50 mg IV.
EPS occur in 0.3% with 10 mg and 0.6% with both 25 mg and 50 mg dosing. Marginal increase in risk of EPS to cover PONV
What are the top 2 therapies for Extrapyramidal symptoms in the PACU?
*Include doses*
-
Anticholinergics
- Benedryl 12.5 - 50 mg IV
- Benztropine
Initial dose: IM, IV, Oral: 1 to 2 mg once.
Subsequent doses: Oral: 1 to 2 mg 1 to 2 times daily.
3-8 all per True Learn but couldn’t find doses
- Trihyexyphenidyl
- Atropine
- Anti-histamines
- Benzodiazepines
- Beta-Blockers
- Dopamine Receptor Agonist
What are all the actions of Diphenhydramine?
Antihistamine (H1)
Anticholinergic activity
Inhibits serotonin reuptake
Potentiates opioid-induced analgesia
May have Local anesthetic-like properties (intracellular sodium channel blocker).
What is the concern for for venous air embolism with Nitrous Oxide?
Although nitrous oxide administration can worsen a venous air embolism, the risk is not increased significantly in intestinal surgery.
When the risk of venous air embolism is high, such as with sitting position neurosurgery, nitrous oxide should be avoided as it can increase the size of an air embolism should it occur.
What are all the contraindications to nitrous oxide?
Answers All in Bold
1. Tension pneumothorax
Expansion of a pneumothorax is the most rapidly occurring of those listed, with volume doubling occurring in only 10 minutes
Expansion of venous air emboli
Worsening of pneumocephalus
Intestinal Obstruction
100cc of gas normally
Expansion could lead to: Not closing, ischemia from lumen obstruction,
Gas-filled spaces = bowel.
One of the concerns with Bowel Distention sx cannot proceed
Blindness
How does chronic opioid use affect the hypothalamic gonadal axis?
Chronic opioid therapy has a profound effect on the adrenal and gonadal axes leading to:
increased prolactin levels (Galactorrhea)
Decreased testosterone, estrogen, cortisol, LH, and FSH.
Pre-renal
BUN:creatinine ratio
FENa
Urine Sodium (UNa)
Urine Osmolality (UOsm)
What are these values?
Pre-renal
BUN:creatinine ratio >20
FENa <1%
Urine Sodium (UNa) <10 mEq/Liter
Urine Osmolality (UOsm) >500 mOsm/kg
What are these values?
Intrinsic
BUN:creatinine ratio
FENa
Urine Sodium (UNa)
Urine Osmolality (UOsm)
What are these values?
Intrinsic
BUN:creatinine ratio <15
FENa >2%
Urine Sodium (UNa) >20 mEq/Liter
Urine Osmolality (UOsm) <350 mOsm/kg
Post-Renal
BUN:creatinine ratio
FENa
Urine Sodium (UNa)
Urine Osmolality (UOsm)
What are these values?
Post-Renal
BUN:creatinine ratio >15
FENa >4%
Urine Sodium (UNa) >40 mEq/Liter
Urine Osmolality (UOsm) <350 mOsm/kg
What is the formula for FENa
FENa = Excreted Na/Filtered Na x 100
(UNa x PCr) / (UCr x PNa x 100)
UNa – urine sodium
PNa – plasma sodium
UCr – urine creatinine
PCr – plasma creatinine
What is the exact molecular mechanism of propofol?
Propofol increases the time that the GABA-A receptors remain open after activation.
GABAA receptors, when active, allow Cl- to enter neurons and hyperpolarize the cell.
Hyperpolarization thus causes increased neuronal inhibition and unconsciousness
Why is it that dexmedotomidine can cause hypertension after bolusing?
In some instances a bolus of dexmedetomidine can cause hypertension because of activation of the sympathetic nervous system.
Alpha2 receptors are prejunctional receptors activated by What?
Alpha2 receptors are prejunctional receptors activated by norepinephrine (negative feedback loop).

The glycine receptor
- What ion channel is linked to this?
- Name an agonist?
- Name an antagonist?
Chloride
- Agonist = Alcohol
- Antagonist = Caffeine
Other than Ketamine, what are some drugs (4) that anesthesiologist can give that promote NMDA channel action?
1. PCP
2. Nitrous Oxide
3. Methadone
4. Tramadol
What is the time frame between doses that places a patient most at risk for bradycardia when administering two separate doses of succinylcholine?
What is the rationale behind this?
However, a second dose of succinylcholine, if given within approximately five minutes of the first dose, increases the risk of bradycardia.
It is thought that the hydrolytic byproducts of succinylcholine metabolism (succinylmonocholine and choline) sensitize the myocardium to the parasympathetic effects of a second dose of succinylcholine, thus leading to bradycardia.
When you simplify ICP, and how we manage this from an intraoperative perspective, what are your options?
1. Drain CSF
2. Decrease parenchymal volume
Diuretics
Steroids
3. Decrease Cerebral Blood Flow
Adjust your PaCO2 with ventilation adjustment
How does CBF pertain to PaCO2?
What is our threshold?
CBF is directly related to PaCO2, and decreasing a patients PaCO2 via hyperventilation is one of the most widely used methods of decreasing CBF and therefore ICP.
CBF changes 1 to 2 mL/100 g/min with each millimeter of mercury change in PaCO2.
Below a PaCO2 of 25 mm Hg, CBF change is attenuated because severe vasoconstriction can cause local ischemia.
Therefore care must be taken when utilizing hyperventilation as a strategy to decrease ICP as ischemia can be an unwanted consequence.
Why is the hyperventilatory therapy for decreasing CBF temporary?
When does this treatment plan not have any more lasting effect?
Ions do not readily cross the blood brain barrier, but CO2 does.
For this reason, acute changes in PaCO2 affect CBF, but changes in blood HCO3- levels have no effect.
However, after 24-48 hours, the CSF HCO3- levels have adjusted to the change, and the CBF returns to normal.
What are the pre-cautions with Methohexital in terms of seizures?
W/ seizure history or epilepsy can trigger a new-onset seizure.
W/O seizure history or epilepsy, methohexital has not been known to trigger a seizures
What are the high dose vs. low dose differences in Phenylephrine effects on?
Arterial vs. Venous constriction
Preload
Cardiac Output
Sphlanchnic Perfusion
Low Dose
Venous constriction results in improved preload from venous return and may improve cardiac output.
This is not seen in patients who have systolic heart failure, as the increased afterload can worsen cardiac output.
Notice VENOCONSTRICTION > arterial constriction
At high dose
Arterial & venous constriction → leads to decreased splanchnic perfusion
In higher doses, CO is commonly reduced due to increases in myocardial work and afterload.
The reduced splanchnic perfusion is more a result of arterial constriction than a reduction in cardiac output.
Why is LMHW more desired than Heparin (High molecula weight)?
(LMWH) more selectively inhibits activated factor X when compared to unfractionated heparin (UFH)
A longer polysaccharide chain (higher molecular weight) is required for the increased binding sites leading to thrombin inhibition.
The shorter polysaccharide chains (lower molecular weight) of LMWH is more restricted in binding solely to ATIII, therefore more selectively inhibits Xa.
Benefit =
1. Selective Xa inhibition can be desirable as factor Xa has limited function outside the coagulation system, unlike thrombin which also plays a role in the immune system and inflammatory pathways.
2. Less HIT
Why would Helium be used in the ICU?
Helium has an extremely low density and thus has an increased tendency for laminar flow.
Helium reduces airway resistance in large and medium airways (reduced resistance in turbulent flow). It can be used to decrease the work of breathing in pathologic conditions.
What is the incidence* and *treatment for Post-Dural Backache (From spinal or epidural)?
Incidence of post spinal backache = 11-13% of patients
Treatment:
Self-Limiting (Resolves in 7 days)
Mild analgesics such as acetaminophen, topical NSAIDs and patient reassurance.
Where are peripheral baroreceptors located?
Peripheral baroreceptors are located in both the:
Common carotid arteries
Aortic arch
How does the baroreceptor mediation occur in High BP vs. Low BP?
(Think about baroreceptors in the common carotid arteries and aortic arch)
When blood pressure is elevated, an increase in the baroreceptor firing in these areas causes an increase in the vagal tone (known as the baroreceptor reflex). (Stretch reflex)
In converse, decreases in blood pressure cause an inhibition of baroreceptor discharge resulting in decreased vagal tone.
When is Infective Endocarditis Prophylaxis Warranted?
1. Include What procedures qualify.
- Include what high risk cardiac conditions apply.
Infective endocarditis prophylaxis is recommended for cardiac conditions listed below PLUS:
-
1) Dental (mucosal, gingival) procedures or
2) Respiratory tract (tonsillectomy, adenoidectomy, bronchoscopy with incision/biopsy) procedures or
3) Infected skin/musculoskeletal tissue procedures* - *High risk cardiac conditions:**
1) Prosthetic cardiac valve or prosthetic material used in valve repair
2) Previous endocarditis
3) CHD only in the following categories: - Unrepaired cyanotic congenital heart disease
- Completely repaired congenital heart disease with prosthetic material or device within six months
- Repaired congenital heart disease with residual defects
4) Cardiac transplantation recipients with cardiac valvular disease
Rank the order of speed of conduction velocity between the different nerve fibers?
Type A - alpha (Fastest), beta, gamma, delta
Type B
Type C - Dorsal Root and Sympathetic (Slowest)
What is the Function of Each of these nerves?
Type A - Alpha
Type A - Beta
Type A - Gamma
Type A - Delta
Alpha = Propiroception , Motor
Beta = Touch, Pressure
Gamma = Muscle Spindles
Delta = Pain, Temp, Touch
What is the Function of Type B nerve Fibers?
Preganglionic Autonomic
What is the function of Type C - Dorsal Root?
Type C - Sympathetic Fibers?
Dorsal Root = Dull pain, Temp, Touch
Sympathetic = Postganglionic
According to the 2016 ACC/AHA guidelines, patients undergoing PCI for stable ischemic heart disease should receive dual antiplatelet therapy for:
What time period after BMS?
What time period after DES?
At least one month after BMS
At least six months after DES.
Contraindications to Metoclopramide?
- SBO (Promotility agent so not ideal)
- Parkinson’s - Increased risk of rigidity and exacerbation of extrapyramidal symptoms
- Pheochromocytoma
What P450 enzyme is affected by St. John’s Wort?
St. John’s wort is an herbal medication that is taken by patients to treat depression. St. John’s wort induces P450 3A4. This induction causes many anesthetic-related drugs to be metabolized including alfentanil and midazolam.
For local anesthetics, what is the more common for anaphylaxis and why?
Amino ester (One “i” in name) anesthetics are degraded into the metabolite para-aminobenzoic acid (PABA).
PABA is the reason for reactions to most local anesthetics.
Anaphylaxis to amino amide (2 “i” in the name) anesthetics occurs at an even lower frequency than in amino ester anesthetics.
Methylparaben, a common preservative, is often found in local anesthetics.
Methylparaben is structurally similar to PABA, and therefore cross reactivity is possible.
In the past, PABA was widely used in sunscreens as a UV filter.
Water-insoluble PABA derivatives such as padimate O are currently used in some products.
When are symptoms of a latex allergy manifested?
Who is at increased risk?
Occurs 30-60 minutes after exposure for allergies but can be immediate if anaphylaxis
More prone = Health care workers, atopic patients, and patients with spina bifida.
What is the one unique complication of a left IJV compared to the right IJV placement?
Increased Chylothorax compared to the right
In the majority of patients the thoracic duct drains into the venous system at or near the junction of the left internal jugular (IJ) vein and the left subclavian vein (SCV)
The most common configuration of the thoracic duct which is found in about 50% of the population involves the thoracic duct originating from the cisterna chyli at around the level of L1, ascending through the abdomen and thorax, and draining into the venous system at the juncture between the left IJ vein and the left SCV.
Variations exist where the thoracic duct drains in the left IJ, left SCV, left brachiocephalic, or left external jugular vein.
Due to the duct’s location, it can potentially be injured during placement of either a central line in the left IJ or in the left SCV.
What volatile anesthetic has been shown to be safest in renal failure patient’s?
The safety of desflurane exposure in patients with renal failure has been shown in various studies.
The potentially nephrotoxic inorganic fluoride concentrations found in humans after exposure to desflurane are significantly less than that after sevoflurane exposure
Unsure about this compared to isoflurane?
Why is Succinylcholine have a black box warning is pediatrics?
Succinylcholine has a Black Boxed warning specifically for this reason.
Caution should also be used in patients with muscular dystrophies (especially in children who can have potentially undiagnosed disorders), stroke patients, patients with neuromuscular disorders, or intensive care patients with prolonged immobilization.
In some patients, such as muscular dystrophy, hyperkalemia can occur secondary to rhabdomyolysis.
What is the timing window in which succinylcholine would not be contrainicated in a burn patient?
<24 hours
It is still appropriate to use succinylcholine to safely secure the airway in these patients if their injury is < 24 hours old, as there has not been enough time to experience nicotinic extrajunctional acetylcholine receptor upregulation.
What is the timeline when succinylcholine becomes harmful with neuromuscular disease?
When is the earliest effect?
When is the latest effect?
Patients with neuromuscular disease such as a stroke have risk of serious hyperkalemia after succinylcholine.
This usually peaks 7-10 days after insult, but increased K+ release may occur as soon as 2-4 days after denervation injury, or after several days of immobility.
Duration of risk has not been adequately characterized but is suspected to be for 3-6 months.
Why is Beta Blocker therapy a Class 1 Level B Recommendation to continue Beta Blockers on patient’s already on them?
A patient who is already on beta blockers should have beta blocker therapy continued in the perioperative period. Continuation of beta blocker therapy is considered Class 1 Level B evidence.
The reason for the continuation of beta-blocker therapy is that beta blocker withdrawal will allow a rebound causing increased heart rate and increased myocardial oxygen demand in the setting of surgical stress.
This increases the likelihood of a MACE
What are the risk factors defined by ACC/AHA when determining whether to start Beta Blocker therapy?
Cardiac risk factors include:
1. Hypertension
2. Cerebral vascular disease
3. Peripheral vascular disease
4. Chronic kidney disease
5. Coronary artery disease
6. Congestive heart failure,
7. Diabetes.
What are the ETT sizes that fit a sit 3-6 Unique LMA?
#3 = 6 mm
#4 = 6 mm
#5 = 7 mm
#6 = 7 mm
What are the ETT sizes that can rescue an iGEL?
#3 = 6mm
#4 = 7mm
#5 = 8mm
What are all the benefits of an epidural for GI surgeries?
Tonic inhibitory sympathetic control (T6-L2) predominates, but parasympathetic activation increases contractility.
Therefore, sympathectomy induced by epidural or spinal analgesia results in increased gut motility, especially those involving epidural catheter placement at T12 or higher.
The gastrointestinal hyperperistalsis has the advantage of providing excellent surgical conditions because of a contracted gut.
Epidural analgesia reduces the duration of postoperative ileus
The use of systemic opioids prolongs the duration of an ileus, and epidural analgesia decreases or eliminates the need for systemic opioids by minimizing postoperative pain.
Less pain also translates into decreased amount of catecholamines released which also promotes better gut contraction.
Postoperative epidural analgesia may also have a protective effect on the gastric mucosa because intramucosal pH is higher during postoperative epidural analgesia than during systemic analgesia.
Local anesthetics, given either neuraxial or systemically, have also been shown to improve intestinal perfusion as well as reduce gastrointestinal tract irritation through their anti-inflammatory properties.
Label all the components of a flow volume loop.

What would Flow-Volume Loop look like for a variable extrathoracic obstruction?

What would Flow-Volume Loop look like for a variable intrathoracic obstruction?

What would Flow-Volume Loop look like for a fixed upper airway obstruction?

What is the best block for tourniquet pain?
- Which nerve?
- Which dermatome origination?
The intercostobrachial nerve provides sensory innervation to the medial brachium and does not originate from the brachial plexus.
It originates from the T2 dermatome.
Blockade of this nerve is required when an upper arm tourniquet is required and would not be successful with any brachial plexus block technique.
What is the sensory and motor innervation of the musculocutanoeus nerve?
How do you block it?
Terminal branch of the lateral cord
Motor: Provides innervation to the biceps muscle (elbow flexion).
Sensory: It gives off the lateral antebrachial cutaneous nerve which provides sensory innervation to the lateral forearm (the site of stimulus) and thus would require supplemental block to achieve adequate anesthesia for the operation.
Block by injecting can be supplemented by injecting local anesthetic into the coracobrachialis muscle.
How is Buprenorphine able to have low physical dependence and prolonged clinical effects?
Buprenorphine has a high affinity for the μ-receptor with a half-life for dissociation from the receptor between 2-3 hours (compare to fentanyl’s 7 minutes).
This leads to a low-level of physical dependence and prolonged clinical effects, up to 6 hours, despite a metabolic and elimination half-life of 3 hours.
What is Buprenorphine potency compared to Morphine?
Buprenorphine has an analgesic potency at low doses that is 25-40 times that of morphine.
What is the cause of a febrile transfusion reaction?
Cytokines and Antibodies to Leukocyte Antigens
What is the cause of graft vs. host disease?
Donor Lymphocytes against a recipient
What is the cause of Delayed Hemolytic Transfusion reaction?
recipient antibody and complement attack on donor cells
result of recipient antibody and complement attack on donor cells
typically secondary to antibodies associated with the Rh, Kidd, or Kell systems
What is the cause of Acute Hemolytic Transfusion reactions?
recipient antibody and complement attack on donor cells
result of recipient antibody and complement attack on donor cells
typically secondary to antibodies associated with the ABO Incompatibility
When should a patient receive leukoreduction with blood products? (What patient population)
Transfusion-related CMV is a problem primarily for:
Immunocompromised patients
Neonates
Allograft recipients
Asplenic from resection or sickle cell disease.
What is the rate of seroconversion with:
HCV
HBV
HIV
HTLV
CMV
1/438 for CMV
1/366,509 for HBV
1/1,657,722 for HCV
1/1,860,883 for HIV
1/3,394,086 for HTLV.
What are the respiratory effects of laparoscopy?
- Decreased lung compliance
- Increased V/Q mismatch
- Increased inspiratory pressures
- Increased PaCO2 and decreased blood pH
What are the cardiovascular effects of laparoscopy?
Cardiac?
Vascular?
- Increased SVR, increased PVR, increased MAP
- Increased cardiac filling (e.g. increased thoracic pressure + head-down position)
- Arrhythmias (tachy or brady)
- Decreased venous return (e.g. vena cava compression + head-up position)
- Decreased ejection fraction
- Decreased renal blood flow
- Decreased splanchnic perfusion
What are the benefits of inhalational induction in an adult?
Benefits:
Stage II (excitation) is generally avoided with high concentrations of sevoflurane used for induction in the adult population.
Sevoflurane also has been administered by mask as an approach to the difficult adult airway because it preserves spontaneous ventilation and does not cause salivation.
The traditional “awake look” in the suspected difficult airway (where intravenous drugs are titrated to a level that allows direct laryngoscopy in the awake patient) has been modified to consist of spontaneous ventilation of high concentrations of sevoflurane until laryngoscopic evaluation is tolerated.
What is the mechanism of action of nicardipine?
Antagonist of calcium influx through the slow channels of cell membranes
Arterial Dilator which decreases afterload and decreases SVR
Coronary Dilator (Angina/Spasms) with minimal effect on preload
Sympathetic activation (25% get tachycardia - NOT Baroreceptor mediated)
Increases cardiac contractility
Nicardipine, nitroglycerin, and sodium nitroprusside all have what in common?
Nicardipine, nitroglycerin, and sodium nitroprusside inhibit hypoxic pulmonary vasoconstriction.
What are some common drugs that have zero order kinetics?
Drugs that typically follow zero-order kinetics are THE PAW: theophylline, heparin, ethanol, phenytoin, aspirin, warfarin.
What is the FENa formula?
FENa = [(PCr x UNa ) / (PNa x UCr)] x 100
How does Uremia lead to impaired platelet function? (Pathophysiology)
Uremia can lead to impaired hemostasis through a variety of mechanisms including, but not limited to:
1. Interference with von Willebrand factor (vWF) formation and release leading to impaired platelet activation at the site of vascular injury. ‘
Normally, platelets are activated and subsequently aggregate by binding to sub endothelial vWF.
Function of glycoprotein IIb-IIIa (GPIIb-IIIa) is abnormal in uremia.
This protein found on the surface of platelets is a receptor for fibrinogen, fibronectin, and vWF, and assists in platelet activation and aggregation.
Prostacyclin and nitric oxide synthesis are each increased in uremia.
These two compounds have platelet inhibitory effects.
Decreased tissue factor (factor III) activity.
This enzyme complexes with factor VIIa to activate factor X to factor Xa which converts prothrombin to thrombin.
What is the common side effect of large volume chloroprocaine through the epidural post-op?
Explain why.
Back pain following a large volume injection of 2-chloroprocaine is not an indication for imaging of the spine.
Back pain following injection of large volumes of 2-chloroprocaine can cause muscle spasms.
2-chloroprocaine may contain the preservative disodium ethylenediaminetetraacetic acid (EDTA).
EDTA is a known chelator of calcium.
Larger volumes of 2-chloroprocaine chelate the calcium of nearby muscle, which causes local muscle spasms.
These spasms are transient and self-relieving.
There is no need for treatment or imaging unless other signs and symptoms are present.
However, there are preservative free formulations of 2-chloroprocaine readily available so this side effect is less commonly seen.
What are the systems based side effects of Amiodarone?
CV: bradycardia, hypotension, Prolonged QTc
Pulmonary: pulmonary toxicity (with a pulmonary fibrosis appearance)
GI: Elevated LFT
Endocrine: hypothyroidism (20% of patients), life-threatening hyperthyroid storm
Dermatological: Blue/Gray Skin and Hypersensitivities
For the pulmonary fibrosis effects of amiodarone, what is thought to cause this?
What is correlated with the severity (Dosing regards)?
Antibody mediated destruction of parenchyma or cytotoxic process
Though the risk of developing side effects rises with the dosage of amiodarone, there is no set dosage limit at which it will occur.
amiodarone-induced pulmonary toxicity correlates more with the total cumulative dose than with the daily dose or plasma concentration.
Metabolism of Amiodarone
Hepatically
What is the difference in metabolism of the amide vs. ester local anesthetics?
Ester type local anesthetic, and like other esters is broken down by plasma cholinesterases.
The amide local anesthetics undergo hepatic metabolism.
What is the reason why local systemic anesthetic toxicity is not seen more with 2-Chloroprocaine?
2-chloroprocaine is so rapidly metabolized and has such low systemic toxicity that it can be delivered in much higher concentrations, up to 3%.
What is primary determinant of potency in local anesthetics?
Lipid Solubility primary determinant of potency in local anesthetics.
What is the primary determinant of duration of local anesthetics?
Protein Binding primary determinant of duration of local anesthetics.
Bier Block:
What is an important potentially devastating consequence?
When should you let down the tourniquet?
What dose and medication should you use?
Pain control is of short duration and stops almost immediately upon deflation of the tourniquet. One important complication of IVRA is systemic toxicity of local anesthetics.
For this reason, it is recommended that the tourniquet not be let down less than 20-30 minutes of local anesthetic injection
The tourniquet should be gradually released to avoid a massive washout of local anesthetic, and long-acting anesthetics such as bupivacaine should be avoided.
Dose = Amount: 40-50 mL of 0.5% Lidocaine (200-300 mg) Example dose would be 12-15 mL of 2% lidocaine (240 - 300 mg) or higher volume of dilute lidocaine
What is the length of time that a Bier Block can last?
Intravenous regional anesthesia (IVRA), also called Bier block, is an alternative to a peripheral nerve block for short (ie, 30 to 45 minutes) procedures on the hand and forearm such as carpal tunnel release, Dupuytren’s contracture release, or reduction of wrist fracture
For non-invasive cuffs, how is the MAP determined?
The point of maximal amplitude of oscillations corresponds to the mean arterial pressure (MAP).
How does the SBP, DBP and MAPs change with non-invasive oscillatory cuff monitoring?
Which is more or less reliable
MAPS = Most reliable
The values of systolic and diastolic pressures are determined using formulas that detect the rate of change in the oscillations.
Thus, systolic and diastolic pressures are less reliable than the mean arterial pressure.
Diastolic blood pressure measurement is more likely to be affected by movement since this would cause changes of similar pressure magnitude, which makes the diastolic pressure the least reliable.
How does succinylchoine affect intraocular pressures?
Intraocular pressure following succinylcholine administration can increase 5-15 mm Hg despite pretreatment with a NDNB.
When you give Succinylcholine, what can be seen with HR?
Cardiac side effects are more often seen with repeat dosing in children (Bradycardia and Asystole) and can be attenuated with the use of glycopyrrolate or atropine.
Succinylcholine more commonly causes tachycardia, mediated by catecholamine release.
You have a patient with an open globe, what do you do for neuromuscular blockade?
Administration of a NDNB can blunt the rise in ICP seen with succinylcholine use. Similar to IOP, inadequate anesthesia, elevated blood pressure, and insufficient neuromuscular blockade during tracheal intubation can elevate ICP higher than succinylcholine alone.
Intraocular pressure following succinylcholine administration can increase 5-15 mm Hg despite pretreatment with a NDNB. The exact mechanism is unknown, but studies have shown that the increase in IOP occurs despite detachment of extraocular muscles.
This has led to the belief that the mechanism most likely is intraocular. Currently, there is widespread recommendation to avoid succinylcholine in open-eye injuries. Importantly, inadequate anesthesia, elevated blood pressure, and insufficient neuromuscular blockade during tracheal intubation can elevate IOP higher than succinylcholine alone.
There is minimal evidence that succinylcholine has led to extrusion of eye contents or blindness.
What nail polish colors affect SpO2 readings?
Blue, green, and to a lesser extent, black nail polish cause falsely low oxygen saturations to be displayed by standard two-wave pulse oximeters by interfering with the differential absorption of 660 and 940 nm light.
What is the dose of methylene blue in Methemoglobinemia?
How does this drug work?
1-2 mg/kg over 3-5 minutes
The drug acts as an electron receptor for NADPH methemoglobin reductase and enhances the enzyme’s effects up to 5-fold, leading to rapid reduction of MetHb to normal Hb.
Methylene blue provides an electron acceptor for the reduction of methemoglobin using NADPH produced from the hexose phosphate pathway.
When you see CaO2 on the ABG, what is this calculating?
CaO2 = [(SaO2)(Hb x 1.34)] + (0.0031 x PaO2)
Where SaO2 is arterial oxygen saturation, Hb is hemoglobin, and PaO2 is partial pressure of arterial oxygen.
How does Insulin secretion change in the perioperative period?
Insulin plasma concentration initially decreases during surgery due to sympathetic activity, which inhibits pancreatic β-cells via α-adrenergic receptors.
Postoperatively insulin concentration usually increases due to hyperglycemia (although the increase is blunted compared non-stressed state)
The stress response also causes insulin resistance.
What are the hormones that decrease in the perioperative period?
T3/T4
GRH
What types of spine conditions is it best to avoid a spinal?
Patients with space-occupying extradural lesions or those that reduce the cross-sectional area of the spinal cord are most at risk for permanent or worsening neurologic injury from a neuraxial anesthetic.
Examples of such lesions include:
Tumors
Severe spinal stenosis
Epidural lipomatosis
Ligamentum flavum hypertrophy
Ependymoma
In these patients, it is generally recommended to avoid neuraxial anesthetic techniques unless the benefits significantly outweigh the risks.
Comment on the risk vs. benefit profile of certain relative contraindications to spinals for:
Multiple sclerosis
Traumatic spinal cord injury
Post-polio syndrome
Diabetic Neuropathy
Relative Contraindications
Some CNS diseases including:
Multiple sclerosis
Traumatic spinal cord injury
Post-polio syndrome only very rarely (<0.3%) develop exacerbation of their disease following neuraxial anesthesia.
Preexisting diabetic neuropathy or other sensorimotor neuropathies have an approximately 0.5-1% incidence of new or worse neurologic deficit following neuraxial anesthesia (higher than the general population).
At high doses, what changes does Meperidine exhibit?
Why is this?
Tachycardia and Hypotension
Increased HR (Chemically similar to Atropine)
Meperidine is structurally similar to atropine and, accordingly, demonstrates atropine-like effects including tachycardia.
Meperidine was initially studied as an anticholinergic agent before being discovered as an analgesic.
The physiologic effects of meperidine differ from other opioids mainly due to two factors:
atropine-like drug effects
weak local anesthetic properties.
At standard analgesic doses, meperidine is associated with hemodynamic stability. However, high doses cause hypotension and tachycardia.
The hypotension is likely attributed to histamine release.
If you give high doses of meperidine and patient becomes hypoventilatory, what is important to know about this?
In addition, high concentrations of meperidine decrease cardiac contractility, an effect resistant to naloxone.
This is due to the local anesthetic effects of meperidine.
How does Nicardipine drop blood pressure?
Effect on afterload? Preload? SVR?
Half Life?
Nicardipine is primarily an ARTERIOLAR vasodilator and decreases left ventricular afterload and systemic vascular resistance with minimal effect upon preload. It is a short acting (t1/2 = 3-14 minutes) dihydropyridine calcium channel blocker that does not significantly decrease cardiac inotropy and may actually cause reflex tachycardia.
How does Neseritide work?
What are then the net effects?
Recombinant form of human brain natriuretic peptide (BNP) which is normally produced by ventricular myocardium.
Brain natriuretic peptide functions as a counter-regulatory hormone to angiotensin II, norepinephrine, and endothelin.
Therefore, nesiritide facilitates cardiovascular fluid homeostasis by down-regulating the renin-angiotensin-aldosterone system, suppressing the sympathetic nervous system, suppressing endothelin production, and stimulating cGMP causing arterial AND venous dilation. Its net effects are vasodilation, natriuresis, and diuresis.
Neseritide Half Life?
When to use?
It has a rapid onset of action and an elimination t1/2 of 15 min.
Nesiritide was once FDA approved for the treatment of acutely decompensated congestive heart failure (CHF).
Recent studies, however, do not recommend its routine use in patients with d ecompensated CHF.
What are the consequences of intraoperative hypothermia?
- Coagulation is impaired by even mild hypothermia which appears to be due to a cold-induced defect in platelet function. This has been shown to increase blood loss in hip arthroplasty.
- Drug metabolism can be impaired by hypothermia, the degree depends on the specific medication and the degree of hypothermia.
- The incidence of wound infection is likely one of the most serious complications associated with hypothermia in terms of morbidity.
- Hypothermia impairs immune function and decreases wound oxygen delivery secondary to thermoregulatory vasoconstriction to that area.
- Patient satisfaction may be negatively impacted by hypothermia as patients often recall unpleasant sensations of being cold in the recovery room; this feeling can last hours even with mild hypothermia. 6. Finally, patient shivering can cause serious discomfort and complications since shivering increases oxygen consumption and can lead to ischemia in at risk patients (resulting in myocardial ischemia and even stroke).
Resting coronary blood flow in the average adult is around 250 ml/min, which represents approximately is what percent of the total cardiac output.?
Resting coronary blood flow in the average adult is around 250 ml/min, which represents approximately 5% of the total cardiac output.
What is the equation for coronary perfusion pressure?
Hence, CPP of the LV (CPPLV) is equal to difference between aortic diastolic pressure (AoDP) and left ventricular end diastolic pressure (LVEDP):
CPPLV = AoDP - LVEDP.
When is the RV:
- Perfused the most?
- Perfused the least?
The RV receives its greatest perfusion during peak/late systole and early diastole and is least perfused during early systole.
How does myaesthenia gravis affect neuromuscular blockade?
Myasthenia gravis is an autoimmune disorder with antibodies against the extrajunctional nicotinic acetylcholine receptor resulting in weakness, enhanced response to nondepolarizing neuromuscular blockade, and resistance to succinylcholine but does not result in upregulation of postjunctional acetylcholine receptors.
Augments Block to Roc
Resistant to Succinylcholine but NOT contra
How fast can a pneumothorax develop when a patient is on nitrous oxide?
A gas mixture of 75% N2O can expand a pneumothorax to double its size in 10 minutes and to 3 times its size in 30 minutes.
What is the relationship of Volatile Anesthetics in terms of:
Blood:Gas Partition Coefficients
Blood Solubility
Uptake
Onset of Action
High BGPC = High Solubility = Greater Uptake = Slower Onset
What is a partician coefficient?
How anesthetic split between two phases (Blood and Gas)
What is the Fa/Fi ratio?
FA / FI = Yields value that can be used to assess relationship of delivered anesthetic by:
Ventilation
Degree of Anesthetic Uptake/Removal
What are the clinical risk factors for CAD?
- *Clinical risk factors for coronary disease include:**
- A history of ischemic heart disease
- Congestive heart failure
- History of stroke
- Diabetes
- Chronic kidney disease.
If a patient has severe COPD, does that preclude them from beta blockade in the perioperative setting?
Severe COPD was once considered contraindication to beta-blocker therapy, however it is no longer considered contraindicated.
Many beta-blockers are cardiac-specific and safe in patients with COPD. The ACC/AHA 2014 guidelines recommend that patient’s on beta-blockers should have them continued through the perioperative period.
Additionally, it is suggested that patients at intermediate or high risk for myocardial ischemia may benefit from perioperative beta-blocker therapy.
What does the ACC/AHA 2014 guidelines recommend for beta blockade in the perioperative setting?
The ACC/AHA 2014 guidelines recommend that patients on beta-blockers should have them continued through the perioperative period. Patients with 3 or more risk factors for coronary artery disease may also benefit from beta-blocker therapy preoperatively.
Clinical risk factors for coronary disease include:
- *- A history of ischemic heart disease
- Congestive heart failure
- History of stroke
- Diabetes
- Chronic kidney disease.**
What is the respective classes and recommendations for:
Class 1 evidence?
Class 2a evidence?
Class 2b evidence?
Class 3 evidence?
- *Class 1** – benefit is greater than risk and recommendation should be followed
- *Class 2a** – benefit greater than risk and it is reasonable to perform therapy
- *Class 2b** - benefit is slightly better or equal to risk and treatment should be considered
- *Class 3** – no benefit and may be harmful
What is the bioavailability depending on route for Midazolam?

Ventilatory Response Curve:
How do surgical stimuli, MAC <1 and Sedative/Hyponotics/Opiates affect this?
Factors that cause a leftward shift and an increased slope of a carbon dioxide ventilatory response curve include arterial hypoxemia, metabolic acidemia, surgical stimuli, and certain CNS pathologies
Volatile anesthetics at ≤1 MAC cause a rightward, parallel shift of the VRC. Volatile anesthetics >1 MAC reduce sensitivity to elevated PaCO2, cause a rightward shift, and reduce the slope of the VRC.
Opioid, barbiturate, and other sedative-hypnotic medication administration shift the VRC to the right and depress the slope, in a dose-dependent manner. The result is a reduced minute ventilation and higher baseline PaCO2 therefore reducing minute ventilation.

How long do you wait for elective surgery for:
Balloon angioplasty?
Bare Metal stent?
No coronary intervention?
Drug Eluding Stent?
14 days after balloon angioplasty
Patients should wait 14 days after balloon angioplasty before elective surgery.
30 days after Bare Metal Stent
Dual Antiplatelet therapy w/ aspirin continuation only in the perioperative period
60 days if no coronary intervention
Acute coronary syndrome patients should wait 60 days prior to scheduling elective surgery.
180 days after Drug Eluting Stent (EVeroliumus eluting stents)for elective non-cardiac surgery
What are the drugs for:
A, B, C, D?

A = Diuretics
B = Vasoildators (ACE-Inhibitors or Nitroglycerine)
C = Milrinone
D = Ionotropes (Epi, Norepi)
What are the most commonly described side effects of spironolactone?
The most commonly described side effects of spironolactone are hyperkalemia, hyponatremia, gynecomastia, and impotence.
Which receptors of Ketamine are used for low dose vs. high dose for analgesia?
The analgesic properties of ketamine are primarily NMDA receptor-mediated at low ketamine doses with proportionally more opioid receptor*-mediated effects at *higher doses.
How does lowering the heart rate increase coronary perfusion?
Lowering heart rate decreases cardiac work since fewer muscle contractions occur over a given period of time. (less ATP thus less oxygen)
- Since the heart is doing less work, there is a corresponding decrease in oxygen demand.
In addition, lowering heart rate will increase the amount of time the heart spends in diastole. Since coronary perfusion occurs primarily during diastole, a longer time spent in diastole improves myocardial oxygen supply. (Time during perfusion)
What is the equation for Coronary Perfusion Pressure?
CPP = (Aortic DBP – LVEDP)
What is the equation to wall tension?
Wall tension = (LVEDP x radius) / (2 x LV wall thickness)
Where: LVEDP = left ventricular end diastolic pressure.
What determines myocardial oxygen supply?
What determines myocardial oxygen demand?
What is a unique variable that determines supply and demand?
Myocardial oxygen supply is determined by the arterial oxygen content of blood, coronary perfusion time, coronary pressure.
Myocardial oxygen demand is determined by myocardial work which is primarily dependent on heart rate and wall tension.
Heart rate is a unique variable in that it plays a role in determining both myocardial oxygen supply and demand.
What type of amnesia is caused by midazolam?
Midazolam causes anterograde but not retrograde amnesia, even at large doses. Typical anxiolysis doses (e.g. 2mg IV in an adult) do not guarantee amnesia.
What do you have to keep in mind about patinet’s who are taking/prescribed oxycodone?
Primarily metabolized to oxymorphone-3-glucuronide and 6-hydroxy-oxymorphone.
The only active metabolite of oxycodone is oxymorphone.
In patients with renal insufficiency receiving oxycodone, the elimination half-life is lengthened and the excretion of metabolites is impaired potentially resulting in a prolongation of its clinical effect.
What are the metabolites of hydromorphone?
- Free Hydromorphone (Accumulates in renal failure)
- Hydromorphone-3-glucoronide (No analgesia and have be neuroexcitatory)
What are the metabolites of Morphine?
Morphine-3-glucuronide (M3G) (55%)
Morphine-6-glucuronide (M6G) (10%)
Normorphine (4%)
Parent compound (10%)
Which metabolite of morphine is problematic for renal failure patients?
Morphine-6-Glucuronide (Active compound: 10%)
What are the 4 major problems with M6G (Morphine - 6 - Glucuronide) accumulating in renal failure?
Respiratory depression
enterohepatic cycling and slow penetration of the blood-brain barrier with equally slow equilibration with systemic circulation; effects are not reversed by dialysis.
Prolonged sedation
Myoclonus
Seizures
What is the major difference in cardiovascular hemodynamics of halothane vs. other volatile anesthetics?
Halothane decreases SBP mainly by reducing cardiac output (NOT SVR)
Other anesthetics = Decrease in SVR, while CO is maintained
Scenario: You use rocuronium for maintenence and then reverse NMB with glycopyrolate and neostigmine.
Patient then requires emergent re-intubation and you use succinylcholine. What do you expect to happen?
Succinylcholine after neostigmine
Following neostigmine administration, a dose of succinylcholine will result in an increased duration of phase I blockade when compared to the duration of action of succinylcholine administered alone.
Phase I augmentation lasts about 30 minutes on average.
Multiple studies have shown that following neostigmine administration for nondepolarizing muscle relaxation reversal, a subsequent dose of succinylcholine will result in prolonged duration of action of the succinylcholine (mean duration 25-35 minutes).
The increase in duration is likely complex and is not solely the result of plasma cholinesterase inhibition.

If you repeat doses of succinylcholine, it will result in a phase II block.
What does this mean?
A phase II block is likely due to the interaction of succinylcholine on the prejunctional acetylcholine receptors.
Prejunctional receptors are blocked by the higher concentration of succinylcholine leading to a decrease in cholinergic transmission and competition with the drug at the postsynaptic receptors. A decrease in plasma cholinesterase activity (either from congenital or acquired causes) can also result in prolonged blockade.
Many medications used for anesthetic maintenance reduce plasma cholinesterase activity including remifentanil, esmolol, ester local anesthetics, and etomidate.

You have an LMA and patient aspirates. First move?
Head-down position
Suction
FiO2 of 100%
Deepen anesthetic
What is the effect of hetastarches on platelets?
Interference with Platelet Adhesion and clot formation by decreasing glycoprotein 2b/3a availability on platelets
Platelets are not able to achieve the appropriate conformation to bind fibrinogen, which negatively affects platelet aggregation
The great radicular artery (aka arteria radicularis magna AKA artery of Adamkiewicz) originates from what level of the spine?
Originates from the aorta between the T9 and T12 vertebral segments in 75% of the population.
If you suspect a spinal cord perfusion problem, what are some treatment options you have?
Spinal Cord Perfusion Pressure (SCPP) Equation
MAP - Intrathecal Pressure or CSF pressure (SCPP = MAP - CSF pressure).
Improve SCPP
1. Increase the MAP to > 90 mmHg (if suspected involvement)
2. Reduce CSF pressure to < 10 mmHg using a lumbar CSF drain.
What are some symptoms of ASA syndrome?
Compromise: Interruption of the great radicular artery → ASA syndrome
Symptoms:
Motor Tract involved
Bilateral lower extremity paraplegia
Bowel and bladder dysfunction.
Dorsal Columns / Sensory NOT involved
Spared Sensation and proprioception (posterior portion of the spinal cord is supplied by two PSAs.)
What is a huge risk of thoracoabdominal Aortic aneursym?
Thoracoabdominal aortic aneurysm (TAAA) repair occurs in 10-20% of patients depending on urgency of procedure, location of the lesion, and duration of ischemia.
Paraplegia in 10-20% of surgeries
With aortic cross clamping, there may be significant hypoperfusion of the spinal cord anteriorly.
What is important to remember about spinal cord perfusion?
Delayed-onset paraplegia is the new onset of neurologic dysfunction several hours to days following surgery in a patient who otherwise woke up without neurologic deficits.
The same principles of SCPP optimization persist and include increasing MAP via vasopressors and/or emergent placement of a spinal drain.
This is an important concept since a spinal cord that was adequately protected during surgery is still at risk for ischemia and infarction postoperatively.
Boards loves to test IICEBALLS in terms of ranking plasma level concentrations of local anesthetics in terms of absorption.
What is this?
IICEBALLS: intravenous > intercostal > caudal > epidural > brachial plexus > axillary > lower limb > subcutaneous
Explain with a mathematical formula how hypoventilation can cause hypoxemia?
Alveolar Gas Equation
PAO2 = FiO2(PB – PH2O) – PCO2 / RQ
*_PAO2_* = alveolar oxygen partial pressure FiO2 = fraction of inspired oxygen PB = barometric pressure PH2O = vapor pressure of water RQ = respiratory quotient
Ex: If PCO2 is 80 mmHg, then your PAO2 would be 50 mmHg.
In healthy patients, minute ventilation will normally increase linearly by approximatley how much for every 1 mmHg increase in PaCO2?
In healthy patients, minute ventilation will normally increase linearly by approximately 2 L/min for every 1 mmHg increase in PaCO2.
What is the equation for volume of distribution?
What is volume of distribution, in theory?
Volume of Distribution (Vd) = Drug dose / Plasma concentration
The volume of distribution does not have an actual physical correlate in plasma volume. Rather, it is the theoretical size of the plasma compartment that would explain the observed plasma concentration if all of the drug given was only distributed in the plasma. Because of this, it is sometimes referred to as the “apparent” volume of distribution.
What does the volume of distribution depend on?
Affinity of the drug for tissue components* (tissue proteins, tissue lipids) relative to its *affinity for plasma components (plasma proteins).
Tissue proteins may include high-affinity receptor sites.
Tissue lipids are an important binding site, depending on the partition coefficient of the drug in fat (and the individual’s fat volume).
What is the volume of distribution directly and indirectly proportional to, respectively?
“The volume of distribution is the total drug within the body divided by the drug plasma concentration.
- It is directly proportional to the unbound drug in the plasma and inversely proportional to the unbound drug in the tissue.
Which drugs have a higher Vd and lower Vd in terms of hydro/lipophilicity and Tissue Binding?
As such, drugs that are lipophilic have a higher Vd than drugs that are hydrophilic.
Also, having greater tissue binding will decrease the unbound proportion in the tissue, thus increasing the volume of distribution.”
What are the indications for cyroprecipitate?
Indications:
- Factor replacement in hypofibrinogenemia
- von Willebrand disease
- Hemophilia A
- Massive transfusion after several units of other blood products have been given. Checking fibrinogen levels during massive transfusion can assist with the management of cryoprecipitate.
How is cryoprecitate made?
What is it high in (substance)?
Synthesis:
Cryoprecipitate is made from fresh frozen plasma
Cooled to 4 degrees Celsius slowly.
Cryoprecipitate is high in factors 8 and 13* as well as *fibrinogen* and *von Willebrand factor (vWF).
How do you dose cyroprecipitate?
ICU vs. OR
Dosing:
It is often given in a pooled dose of 10 or more units.
Concentration:
- Contains approximately 200 mg/unit of fibrinogen.
- 1200 mg/dL: 1 unit is 12.5cc
Therefore, administration of 10 units would add 2 gm of fibrinogen.
1. ICU
Ten units of cryoprecipitate (2 grams) will typically raise a 70kg patient’s fibrinogen by 70 mg/dL
2. Intraop
FIBTEM A10 <10mm
Goals (Ask surgeon if we are acutely bleeding or oozing)
- Acute Hemorrhage = We need FIBTEM A10 ~ 18 mm
- Oozing = We need FIBTEM A10 ~ 9 mm
Treat with Fibrinogen (2 mm FIBTEM A10 increase with 1000 mg of Fibrinogen)
What are the three nerves provide sensory and motor innervation to the airway?
- Glossopharyngeal
- Recurrent laryngeal
- Superior laryngeal

What airway structures are innervated by CN IX? (glossopharngeal)
Base of Tongue
Vallecula
Anterior Epiglottis
Tonsils

How do you anesthetize the glossopharngeal nerve for awake fiber intubation?
Nerve Block
Topical application of local anesthetic in the gutters of the pharyngeal folds near the base of the tongue for 5-10 minutes or with injection of local anesthetic at the base of the fold in the floor of the mouth.

What do you have to watch out for in terms of glossopharyngeal nerve anesthetizing during awake fiber intubation?
Watch out for carotids!
If blood aspirated, needle redirection medially should occur.
The glossopharyngeal nerve is the sensory nerve associated with the gag reflex
What are the motor and sensory innervations of the Recurrent Laryngeal nerve?
Motor and Sensory nerve, motor involved with cough reflex
Primary motor nerve of the larynx
Sensory innervation to the vocal folds and trachea

How do you anesthetize the recurrent laryngeal nerve?
Anesthesia of this nerve is provided by transtracheal injection of local anesthetic after air is aspirated with puncture through the cricothyroid membrane or through aerosolization of local anesthetic and having the patient breathe it in.

What are the two branches of the superior laryngeal nerve?
Internal and External Branch
What are the innervations of the internal branch of the superior laryngeal nerve?
Base of the tongue
Epiglottis
Aryepiglottic folds
Arytenoids
How do you block the internal branch of the superior laryngeal branch?
It can be blocked by cotton soaked in local anesthetic applied with a right-angled clamp into the piriform sinuses for 5-10 minutes but is often blocked by initial topicalization of the pharynx.
Injections can also be performed after walking off the cornu of the hyoid bone through the thyrohyoid membrane

What is the innervation of the external branch of the superior laryngeal nerve?
Motor innervation to the cricothyroid muscle, the only laryngeal muscle not innervated by the recurrent laryngeal nerve.

What are the 3 ways machines can measure oxygen?
Clark, galvanic, and paramagnetic electrodes measure oxygen
What anesthesia machine measures CO2?
Severinghaus electrode measures CO2
What anesthesia machine measures pH?
Sanz electrode measures pH
What is the equation for Coronary Perfusion Pressure of the Left Ventricle?
CPPLV = AoDP - LVEDP
Hemodynamic variables that increase LVEDP will decrease CPP.
What is our proxy of LVEDP in cardiac surgery?
Pulmonary artery occlusion pressure (PAOP) may be used as a proxy for LVEDP. A decrease in PAOP is generally regarded as a decrease in LVEDP, which leads to an increase in CPP.
What is the equation of Coronary Perfusion Pressure of the LV?
Aortic Diastolic Pressure - LVEDP
What are the contraindications for LMA placement?
(List generalized groups to help remember)
Habitus (BMI >30)
Gastroesophageal
High risk for aspiration of gastric contents including
Known or potential for full stomach (NOT NPO)
Hiatal hernia
Gastroesophageal reflux disease
Intestinal obstruction
Delayed gastric emptying
Pulmonary
Poor lung compliance
High airway resistance
Airway
Difficult Airway
Glottic or subglottic airway obstruction
Limited mouth opening (generally less than 1.5 cm).
What is the 11-beta hydroxylase enzyme responsible for converting?
(This is the enzyme blocked with etomidate)
The 11-β hydroxylase enzyme converts deoxycorticosterone to corticosterone and 11-deoxycortisol to cortisol.
What phenomenon states that oxygen is more readily released from hemoglobin in the face of acidosis or hypercarbia?
Bohr Effect

What ar the bioavailabilities for Midazolam dependent on route?
Midazolam bioavailability among routes of administration (greatest to least):
intravenous > intramuscular > intranasal > rectal > oral.
Oral bioavailability is highly dependent on dose; bioavailability is lower with higher doses.
This means that rectal bioavailability could potentially be lower than oral, but again it is dose dependent.

How do we rank PONV risk in pediatrics?
- >3 years
- Duration of surgery (>30 minutes)
- Type of Procedure (Strabismus and T&A)
- Patient or family history of PONV
Zero, 1, 2, 3, or 4 of these risk factors impart a risk of postoperative vomiting of 9%, 10%, 30%, 55%, and 70% respectively in pediatric patients.
What is the pediatric dose for PONV of:
Zofran and Dexamethasone
Zofran 0.1 - 0.15 mg/kg
Dexamethasone 0.15 mg/kg
What could potentially happen when you overdose neostigmine?
Give some of the pathophysiological reasoning.
Weakness
Reversal of nondepolarizing neuromuscular blockade is achieved by administering an AChE inhibitor. (Neostigmine).
NMBD = competitive antagonists and compete with acetylcholine (ACh) in the synaptic cleft. The chances that ACh will bind to ACh receptors (instead of the NMBD) increases with AChE inhibition (Neostigmine)
Excessive inhibition of AChE can lead to prolonged elevations in synaptic ACh concentrations.
- Prolonged exposure to ACh can lead to both presynaptic and postsynaptic inactivation of sodium channels.
- Inactivation of sodium channels prohibits depolarization from occurring. 3. In addition, both presynaptic and postsynaptic ACh receptors become desensitized to ACh.
Restrictive Disease with PFT will show what?
FVC
FEV1
FEV1/FVC
FEF 25-75%
FRC
TLC
Restrictive Disease
Reduced diaphragmatic excursion leads to decreased lung volumes
FVC - Low
FEV1 - Low
FEV1/FVC - Normal or even high
FEF 25-75% - Normal
FRC - Low
TLC - Low
Obstructive Disease with PFT will show what?
FVC
FEV1
FEV1/FVC
FEF 25-75%
FRC
TLC
Obstructive Disease with PFT will show
FVC - Normal or Increased
FEV1 - Decreased
FEV1/FVC - Decreased
FEF 25-75% - Decreased
FRC - Normal or increased
TLC - Normal or increased
What is the normal range for FEF 25-75% and what does this mean?
The forced expiratory flow during the middle 50% of the FVC (FEF25-75%)
another way of saying this
Forced expiratory flow (FEF) is the flow (or speed) of air coming out of the lung during the middle portion of a forced expiration
Normal ranges for FEF25-75% are typically 80-120% of the predicted value.
Wikipedia
Values ranging from 50-60% and up to 130% of the average are considered normal
Why can Ketorolac interfere with renal function?
Ketorolac can decrease GFR, especially when administered for greater than 5 days, via inhibition of afferent renal artery vasodilation.
Blocks prostaglandins which vasodilate the afferent renal artery.
What is the difference between a Type and Screen and Type and Cross?
Both = Screen for ABO, Rh, Antibody
T&C = Donor RBC and patient serum for antibody screening

Which antibiotics decrease ACh release?
Factors that decrease release of acetylcholine:
1) Antibiotics (clindamycin, polymyxin)
How does magnesium and calcium levels affect Ach release?
Factors that decrease release of acetylcholine:
- *Magnesium**: antagonizes calcium
- *Calcium**: Hypocalcemia
How do anti-epileptics affect ACh release?
Factors that decrease release of acetylcholine:
Anticonvulsants
How do diuretics affect ACh release?
Factors that decrease release of acetylcholine:
Diuretics (furosemide)
How does Lambert Eaton and Myasthenia Gravis affect ACh release?
Factors that decrease release of acetylcholine:
Eaton-Lambert syndrome: inhibits P-type calcium channels
Myasthenia gravis is due to antibodies destroying post-synaptic acetylcholine receptors. Acetylcholine release from nerve terminal is not affected.
How does Nifedipine and Diltiazem affect ACh release?
Calcium channel blockers (nifedipine, diltiazem) do not affect P-channels. These drugs affect L-type calcium channels.
What are the similarities and differences between Botulism and Tetanus?
Both the botulinum and tetanus toxin inhibit SNARE proteins.
However, botulinum* toxin acts at the level of the *peripheral nerve, and the tetanus toxin acts at the central nervous system.
Botulinum –> inhibits SNARE –> decrease acetylcholine release (peripheral nerve)
Tetanus –> inhibits SNARE –> inhibits GABA neurons (CNS)

What are the major independent preoperative risk factors for postoperative AKI following noncardiac surgery in patients with normal renal function?
“BLEACH P”
- Age ≥ 59
- BMI ≥ 32
- Chronic Liver disease
- COPD requiring chronic bronchodilator use
- Peripheral vascular occlusive disease
- High-risk surgery
- Emergency surgery
What is the definition of FRC?
FRC is the capacity in the lungs after end expiration of a normal tidal volume and consists of the expiratory reserve volume (ERV) and the residual volume (RV).

What is the definition of Closing Capacity?
Closing capacity is the capacity during which airway closure begins to occur and consists of the closing volume and residual volume.

What are the 6 most common things that decrease FRC?
A mnemonic for factors that decrease FRC is PANGOS:
Pregnancy
Ascites
Neonate
General Anesthesia
Obesity
Supine position.
What are the 5 most common things that increase closing capacity?
A mnemonic for factors increasing closing capacity is ACLS-S:
Age
Chronic bronchitis
LV failure
Smoking
Surgery
What is the most important factor when considering Pancuronium?
Pancuronium is eliminated primarily by the kidney and therefore should be avoided in renal failure.
Glucagon is generally not considered a first line therapy for low cardiac output (CO) states, but may be beneficial in which following 5 situations after conventional therapies have failed?
Glucagon is generally not considered a first line therapy for low cardiac output (CO) states, but may be beneficial in the following situations after conventional therapies have failed:
1. Low CO following cardiopulmonary bypass (CPB)
2. Low CO following myocardial infarction
3. Chronic congestive heart failure
4. Low CO from excess β-blockade
5. Anaphylactic shock with refractory hypotension
What is the dosing of Glucagon for Anaphylaxis vs. Beta Blocker Overdosing?
Anaphylaxis (refractory to epinephrine) in patients on beta-blocker therapy (off-label use): IV:
Initial: IV 1 to 5 mg bolus
Followed by an infusion of 5 to 15 mcg/minute
Titrate the infusion rate to achieve an adequate clinical response (Lieberman 2015).
Beta-blocker overdose
IV: 3 to 10 mg bolus; if no clinical response may repeat bolus dose; if clinical response with bolus, start continuous infusion at 3 to 5 mg/hour;
titrate infusion rate to achieve adequate hemodynamic response (ACC/AHA/HRS [Kusumoto 2018]; AHA [Vanden Hoek 2010]; Barrueto 2019).
If you see a patient taking St. Johns Wort, whata are some important factors in this?
This patient is taking St. John’s Wort which induces cytochrome P450 isoenzymes and increases the metabolism of cyclosporine.
This can result in subtherapeutic drug levels and an i_n_creased risk of transplant rejection
Warfarin therapy is therefore unreliable in patients taking St. John’s Wort and puts them at risk for DVT and thromboembolism.
What characteristic of an opioid is a prime determinant of the onset and duration of action of the drug?
The lipid solubility of an opioid is a prime determinant of the onset and duration of action of the drug as it affects how easily the drug is able to cross cellular (lipid) membranes.
How does the pKa of an opiate affect its function?
An opioid with a pKa less than physiologic pH (~7.4) will have a much greater nonionized fraction whereas an opioid with a pKa >7.4 will have a greater ionized fraction.
Lower pKa = Increased non-ionized fraction = Faster onset
How does Obesity manifest on PFT’s?
Both FEV1 and FVC are decreased proportionally so that the FEV1/FVC ratio is preserved, i.e. obesity behaves like a restrictive defect.
What is your delayed emergence checklist?
O DAMN
O = Oxygen
D = Drugs (Vapor, Sedative/Hyponotic/NMBD/Atypical Pseudocholinesterase, Benzo,)
- Antagonism with Naloxone, Nalbuphinie, Sugammadex, Physostigmine (For Scopalamine), ETOH or intoxication
Altered Metabolic Derangments
- Hypoglycemia
o Assess blood glucose level - Hypoxemia and/or hypercarbia
o Assess vital signs including pulse oximetry and end tidal CO2.
o Obtain arterial blood gas. Continue mechanical ventilation until corrected. - Hypothermia/hyperthermia
o Assess vital signs including temperature (E) - Acidosis
o Obtain arterial blood gas and correct underlying disorder as necessary -
Hyponatremia (e.g. after urologic surgery)
o Obtain metabolic panel and correct as necessary
N = Neurological insults
- New stroke, either ischemic or hemorrhagic
o Perform full neurologic exam if able (pupils, cranial nerves, reflexes, withdrawal to pain)
o Consider CT scan or MRI scan if adequate concern or suspicion - Seizure or post-ictal state
- Increased intracranial pressure
What is your delayed emergence rapid panel?
Delayed Emergence Rapid Panel
Vital signs (including temperature)
Twitch monitor
Neurologic Exam (pupils, cranial nerves, reflexes, response to pain)
Fingerstick glucose
ABG with electrolytes
Make arrangements for naloxone, flumazenil, physostigmine, imaging (ex. CT scan)
What is your dosages for drugs during delayed emergence?
Included under drug effects are:
Residual anesthetic (volatile, propofol, barbiturates, ketamine)
Excess narcotics – can be reversed by naloxone (40 ucg boluses) – remember it’s short-acting
Preoperative sedatives – too much midazolam? – reversed by flumazenil 0.2 mg q1min up to 1 mg
Physostigmine 1.25 mg IV can reverse cholinergic effects (ex. scopolamine) and possibly the effects of anesthetic agents (Stanford Delayed Emergence Protocol)
Inadequate reversal or no reversal of muscle relaxation or rarely pseudocholinesterase deficiency – edrophonium/atropine work faster (1-2 mins) than neostigmine/glycopyrrolate (peak effect around 10 mins) and may be indicated in this setting
Acute alcohol intoxication or other illicit drugs rendering unconsciousness extending the length of the anesthetic
Where should you place the black and red electrodes when monitoring NMB?
Ulnar: Black is Distal, Red is proximal
Face: Black is aligned with tragus, Red is above

What is the best muscle to determine if abdominal muscles are paralyzed?
Corrugator Supercilii

Where should you monitor neuromuscular blockade on induction and on emergence?
Induction = Corrugator Supercilii
It is the best comparison for abdominal muscle paralysis as the diaphragm and abdominal muscles are also relatively resistant to neuromuscular blockade. These muscles are the first to recover from neuromuscular paralysis. Corrugator supercilii monitoring is reflective of laryngeal muscle paralysis and may be used to assess for adequate intubating conditions.
Emergence = Adductor Pollicis
The adductor pollicis muscle is innervated by the ulnar nerve and causes adduction of the thumb. The adductor pollicis is more sensitive to neuromuscular blockade than the corrugator supercilii. Therefore the adductor pollicis is less representative of abdominal muscle paralysis. The adductor pollicis is a commonly monitored muscle to assess neuromuscular blockade as it is often accessible, visible, and correlates well with recovery of laryngeal muscle function during extubation. A primary benefit of monitoring the adductor pollicis is upon emergence.
100Hz for 5 seconds of Tetanus correlates to about a TOF ratio of what if sustained?
100Hz for 5 seconds correlates to about a TOF ratio of 0.85 if sustained.
These tests correlate with what TOF ratio?
MIP > 25 cm H2O, vital capacity > 15 mL/kg, tidal volume assessment, sustained eye-opening, handgrip, and tongue protrusion.
These tests correlated with a what TOF ratio?
MIP > 50 cm H2O, head-lift test, leg-lift test, tongue depressor test, and handgrip (sustained). There are no clinical tests available to date that can assess for a TOF ratio of ≥ 0.9.
1: TOF <0.7
2: TOF 0.7 - 0.9
ETT dosing:
What is the dose of epi during cardiac arrest?
2-2.5 x the IV dose (2.5 mg diluated in 5-10 mL of sterile water)
What is the equation used to determine how much FFP you need?
An equation for this is: Amount FFP Needed (mL) =
(Target Level - Present Level) * kg
With each INR, what is the respective Vit K-dependent factor activity?
- 0
- 4-1.6
- 7-1.8
- 9-2.1
- 2-2.5
- 6-3.2
- 0-4.9
>5.0
- 0 100
- 4-1.6 40
- 7-1.8 30
1.9-2.1 25
- 2-2.5 20
- 6-3.2 15
4.0-4.9 10
>5.0 5
How much FFP would be needed for a 70kg patient who is bleeding with an INR of 7.5 and our goal INR is 1.4?
For example, let’s say we have a 70kg patient who is bleeding with an INR of 7.5 and our goal INR is 1.4.
INR of >5 = 5% activity
INR of 1.4 = 40% activity
We would need (40% - 5%) * 70 kg, which is 2450 mL of FFP to make this correction. A single unit of FFP typically is 200-250 mL.
Starting INR of:
- 5
- 0
and 1.8
How many units of FFP are needed to reach the INR of 1.5?
- 5 4 units
- 0 3 units
- 8 2 units
How does paraplegia or quadriplegia affect neuromuscular blockade monitoring?
The proliferation of extrajunctional acetylcholine receptors in muscle in paralyzed limbs can lead to an increased response (T4:T1) to peripheral nerve stimulation following non depolarizing neuromuscular blockade compared to a normal non paralyzed limb in the same patient.
Whta are the 4 potassium sparing diuretics?
EAST
Eplerenone
Amiloride
Spironolactone
Triamterene
What is the Fick Equation to determine if SvO2 increases or decreases?
SaO2 - [VO2 / (CO * Hgb * 4/3))]
How long do you have to wait for non-cardiac surgery for patients who had MI for:
Ballon Angioplasty
Bare Metal Stent
Drug Eluding Stent
No intervention
14 days after balloon angioplasty
30 days after Bare Metal Stent
60 days if no coronary intervention
180 days after DES for elective noncardiac surgery.
What is your differential for Increased peak inspiratory pressure and increased plateau pressures?
Pulmonary Complianace (Elastic Resistance)
Positioning
Abdominal Insufflation
Decrease pulmonary compliance
Trendelenburg Position
Intrinsic Disease
Ascites
Intrinsic Lung Disease
Obesity
Acute Events
Pulmonary Edema
Tension Pneumothorax
You have a patient with elevated peak pressures.
What is your differential and first steps?
Two things can be happening when the peak inspiratory pressure is high
Peak pressure is high with a normal plateau pressure, indicating a problem with resistance
Peak pressure is high with an elevated plateau pressure (normal < 25 cm H2O) demonstrating that there is a problem with lung and/or chest wall compliance

What is your differential for Increased peak inspiratory pressure and normal plateau pressures?
Problem is with airway resistance
- Airway compression
- Bronchospasm
- FBO
- Kinked ETT tube
- Mucuous Plug
- Secretions

What is the normal stroke volume and stroke volume index?
Stroke Volume (SV) 60 - 100 mL/beat
Stroke Volume Index (SVI) 33 - 47 mL/m^2/beat
What is Cardiac output in terms of Blood Pressure and SVR?
CO = BP/SVR
What is EMLA?
When is it contraindicated?
EMLA is a local anesthetic mixture applied transdermal to provide anesthesia for the skin.
Eutectic Mixture of Local Anesthetics (EMLA)
EMLA contraindications include:
1. Allergy to amide anesthetics
- Concomitant class III anti-arrhythmic drugs (e.g., amiodarone, sotalol)
- Congenital or idiopathic methemoglobinemia, infants receiving treatment with methemoglobin-inducing agents.
- G6PD
What is a shortcut for calculating the volume of liquid anesthetic consumed in one hour?
Liquid volatile anesthetic (mL/hr) ≈ 3 * FGF (L/min) * % anesthetic vapor.
OR
- Explain what happens in acute normovolemic hemodilution is.
2. What are the physiological consequences?
Involves giving fluid and intentionally diluting the patient’s blood prior to significant blood loss.
Blood is withdrawn and stored for later autologous transfusion, and the patient receives the same volume in crystalloid or colloid to maintain euvolemia.
Physiological Consequences:
Blood viscosity will decrease.
The decreased blood viscosity will decrease peripheral vascular resistance
Cardiac output raises to compensate for the anemia.
The increase in cardiac output and decrease in peripheral vascular resistance causes an increase in regional blood flow.
However, even with increases in regional blood flow and cardiac output, there is no increase in oxygen delivery. This is secondary to the significant amount of oxygen that hemoglobin can carry.
Hgb of 7-8 is targeted
Consequently, blood is able to move with less resistance because of the reduced viscosity, and thereby a drop in peripheral vascular resistance occurs with an increase in regional blood flow.
If cardiac output can effectively compensate, oxygen delivery to the tissues at a hematocrit of 25% to 30% is as good, but no better than, oxygen delivery at a hematocrit of 30% to 35%.
Give the % of Glucose vs. % Ketone Bodies for brain energy in terms of time:
Normally = ?
Fasting = ?
3 days of Fasting = ?
30-45 days Fasting = ?
Under normal circumstances, the brain uses glucose as its sole energy source (No ketone bodies)
During periods of fasting, exercise, or very low carbohydrate diets, the brain will begin to use ketone bodies (acetone, acetoacetic acid, and β-hydroxybutyric acid) as an alternative source of energy.
After 3 days of low blood glucose, ketone body metabolism supplies approximately one-third of the brain’s energy.
After 1-1.5 months, they supply two-thirds of the brain’s energy. Glucose continues to account for the remainder. The increased use of ketones decreases the body’s demand for glucose which allows for decreased catabolism of muscle for conversion of amino acids to glucose.
What is the sodium concentration in 5% albumin?
The sodium concentration of standard 5% albumin manufactured in the United States is 145 +/- 15 mEq/L.
What are the differences in pain that will present for:
Intraperitonteal perforaton vs. extraperitoneal perforation?
Extraperitoneal perforation (the most common) causes periumbilical, inguinal or suprapubic pain, whereas intraperitoneal perforation causes diffuse or upper abdominal pain, or referred pain to chest and shoulders.
What is TURP syndrome?
As a side note, keeping the patient awake has the additional benefit of allowing for the detection of TURP syndrome, which is due to hypo-osmolality and hyponatremia and presents with CNS symptoms.
How does Glycine toxicity manifest itself?
Glycine toxicity causes hyperammonemia, which manifests as CNS symptoms and nausea. Transient blindness can also occur.
What is the onset* and *duration of neostigmine?
Onset = 7-11 minutes
Duration = 4-6 minutes
What is important to note about PCP in terms of their anesthetic?
Reduced MAC requirements
What are the top 3 factors that lead to death and permanent brain damage?
The top 3 factors leading to death and permanent brain damage are:
Cardiovascular events (pulmonary embolism, stroke, myocardial infarction, arrhythmia, undiagnosed conditions)
Respiratory events (inadequate ventilation, esophageal intubation, difficult airway)
Equipment issues (failure or misuse)
Listed is N2O Concentrations:
50% N2O
75% N20
80% N20
Whaat % of bowel distention is present after 4 hours for these respective concentrations?
N2O Concentration Bowel Distension After 4 Hours
50% N2O
200% increase
75% N2O
400% increase
80% N2O
500% increase
How does coronary dominance affect AV node perfusion?
In patients with a coronary supply that is right-dominant, the RCA also gives rise to the AV nodal artery which supplies the AV node.
The most common cause of AV node disruption and resulting complete heart block is a myocardial infarction, most frequently a blockage in the RCA.
What determines SA node perfusion?
The SA node is supplied by the RCA in about 60% of cases and by the LCA or its branches in about 40% of cases.
Supply of the SA node does not depend on coronary dominance of the patient.
What is the treatment for myxedema coma?
Thyroid Replacement Therapy
Levothyroxine is the preferred therapy because it allows the controlling enzyme systems to regulate TSH secretion as well as conversion to T3.
Liothyronine, the most potent form of thyroid hormone, can be given intravenously though it risks precipitating myocardial ischemia.
Hydrocortisone may also be given because 5% to 10% of patients with myxedema coma also have adrenal insufficiency.
What are the screening criteria for OSA?
How do we interpret these scores?
- *S**noring
- *T**ired during the day
- *O**bserved apneas
- *P**ressure (high blood pressure)
- *B**MI > 35
- *A**ge >50
- *N**eck size
- *G**ender (male)
<3 = Low risk of OSA
≥3 = High risk of OSA
How is the polysomnogram interpreted?
Sleep studies are scored with an apnea/hypopnea index (AHI).
Mild OSA is defined as 5-15 events/hour of sleep (AHI 5-14).
Moderate OSA is defined as 15-30 events/hour of sleep (AHI 15-29).
Severe OSA is defined as greater than 30 events/hour of sleep (AHI 30+).
What are the intermediates of sevoflurane and how does that relate to hepatic consequences?
Sevoflurane metabolism has not resulted in the formation of TFA intermediates and the potential for hepatic toxicity is low.
Instead, sevoflurane metabolism yields a compound hexafluoroisopropanol (HFIP), which does not accumulate and rapidly undergoes phase II biotransformation.
This contributes to the low hepatotoxic potential for sevoflurane.
What are the two types of postoperative liver injuries with Halothane?
Two types of postoperative liver injuries have been reported to be associated with halothane administration.
The first type is a mild injury characterized by nausea, lethargy, and fever.
The second type, mediated by the patient’s immune system, is severe acute hepatitis with histological findings of widespread hepatic necrosis. This is rare (1 in 15,000 cases on initial exposure) and associated with acute elevations in ALT and AST and onset of jaundice within 2 to 14 days of halothane administration.
What are the intermediates of Desflurarne?
Desflurane, as a consequence of trifluoroacetic acid (TFA) reactive intermediates, has been implicated in the formation of dangerous immunogenic compounds.
How would you explain how CO2, Voltaile anesthetics, and N2O is interpreted by a machine?
It operates on the principle that the amount of infrared light absorbed at a specific wavelength is proportional to the partial pressure of the gas analyzed because absorption will be greater with a greater amount of gas molecules.
Intensity of IR light detected will therefore be inversely proportional to the amount of gas in the sample.
What is the renal excretion % of Rocuronium?
How is it primarily cleared?
Approximately 25-30% of rocuronium is renally excreted.
It is cleared primarily by hepatic uptake* and *hepatobiliary excretion.
At what rate of transfusion is required in order to obtain citrate intoxication?
Citrate intoxication occurs with massive transfusions, most notably with fresh frozen plasma.
The rate of transfusion needs to exceed 1 mL/kg/min, which is approximately equivalent to one unit of packed red blood cells (PRBCs) every 5 minutes in an adult.
In the setting of citrate intoxication, what are the hemodynamic and EKG parameters that are seen?
Hemodynamics - Hypotension, Narrowed Pulse Pressure, Increased LVEDP (Decrease in Coronary Perfusion Pressure)
EKG - Prolonged QT, Narrowed QRS, Flat T waves
What are the components of the CPDA-1 anticoagulation in PRBC?
CPDA-1 anticoagulant allows PRBC and whole blood storage for up to 35 days
- Citrate is the anticoagulant (binds calcium necessary for clot formation)
- Phosphate is incorporated for cellular function and ATP production
- Dextrose is the nutrition source for glycolysis
- Adenine is incorporated for ATP production
Why is CPDA-1 used in PRBC rather than EDTA?
EDTA is an irreversible binder of calcium
What is the level of responsiveness in the depth of sedation continuum?
Minimal Sedation - Normal rersponse to verbal
Moderate sedation - Purposeful rersponse to verbal/tactile
Deep - Purposeful response after repeated/painful
General anesthesia - Unarousable
Other than nitrous oxide, what is another gas cylinder that should be weighed to determine its fullness?
Carbon Dioxide
What is the color of CO2 tank?
Gray
What is the color of Helium tank?
Brown
When will a Nitrous Oxide tank begin to drop pressure?
How much does a full Nitrous Tank weight?
An empty nitrous tank?
~400L (25% full)
A full E cylinder of nitrous oxide is 8.80 kg and has a pressure of 745 psig, an empty cylinder is 5.90 kg.
How do you differentiate a Grade III view?
Grade III: no cords visible, only epiglottis visible.
- *Grade IIIa: only epiglottic edge visible (epiglottis** raised).
- *Grade IIIb**: downfolded or floppy epiglottis is visible.

What is the normal gradient between PaCO2 and ETCO2?
Does it change with anesthesia?
PaCO2 is always greater than ETCO2 with a gradient of 2-5 mm Hg in spontaneously breathing, nonanesthetized patients
Yes. under anesthesia = 5-10 mm Hg in mechanically ventilated patients.
Ex: ETCO2 is 40, you will have PaCO2 of 45-50 mmHg
Why does the Desflurane Vaporizer need to be heated to 2 atm?
This is because desflurane is near boiling point at room temperature (73 F), which means an unpredictable amount is in liquid form, while the rest of it may be in gaseous form.
This heated vaporizer reliably converts desflurane to the gaseous form.
What is the reason Ativan (Lorazepam) is useful in renal disease?
5 metabolites - but primarily undergoes glucuronide conjugation without any phase I metabolism and, because of this, is less affected by age and renal disease
The main metabolite of lorazepam is inactive and water-soluble, thus is excreted rapidly by the kidneys
What are the most common side effects of Ondansetron? (List 4)
QTc prolongation (20%)
Headache (11%)
Transient transaminitis (5%)
Constipation (4%)
Ondansetron causes QTc prolongation.
Should we avoid this medication? Why or why not?
Ondansetron is known to cause QTc prolongation in nearly 20% of patients. However, the prolongation is typically 20-30 msec or less, which is clinically insignificant (though statistically significant)
except in patients with already prolonged QTc intervals or those with additional risk factors for prolonged QTc intervals (e.g. hypokalemia, hypocalcemia, hypothyroidism, history of myocardial infarction, long QT syndrome, and recent use of other medications that prolong the QTc interval).
If you have a patient with Prolonged QT syndrome, what adjustments should be made during their anesthetic?
TIVA (Vapor can prolong QTc)
Correct Potassium (Hypo can exacerbate)
Correct Calcium (Hypo can exacerbate)
Get TSH and Free T4 levels (Hypo can exacerbate)
What are the criteria for determining if prophylaxis against infective endocarditis is necessary?
HIGH RISK FACTORS + HIGH RISK PROCEDURES ARE REQUIRED
- *The high-risk factors are:**
1) Prosthetic heart valves
2) Prior history of infective endocarditis
3) Unrepaired cyanotic congenital heart disease
4) Completely repaired congenital heart defect (the first six months after procedure)
5) Repaired congenital heart disease with residual defect
6) Valvular disease in a transplanted heart
Of note, prophylaxis is NOT required in patients with bicuspid aortic valve, acquired aortic/mitral disease, or hypertrophic cardiomyopathy (HOCM).
-
The high-risk procedures are:*
1) Dental work – highest risk for infective endocarditis in procedures that involve gingival tissue manipulation or perforation of the oral mucosa. For example, tooth extractions, or drainage of a dental abscess. However, routine dental cleaning is not a high-risk procedure that requires prophylaxis.
2) Respiratory tract procedures that warrant prophylaxis are those involving incision/biopsy of the respiratory tract mucosa. This includes tonsillectomy, adenoidectomy, and bronchoscopy with biopsy.
3) Skin or musculoskeletal tissue procedures
You determine a patient required antibiotic prophylaxis, what is the regimen you will put them on perioperatively?
The current prophylactic regimen is:
- Amoxicillin
OR
- Ampicillin 2 g given 1 hour before the procedure.
You have a pencillin allergy to a patient that needs infective endocarditis prophylaxis. What do you prescribe?
Those who are allergic to penicillin can be given clindamycin 600 mg.
OR
Azithromycin 500mg or ceftriaxone 1g
What is the order of NMBD potentiation among volatile anesthetics?
The order of non-depolarizing muscle relaxant potentiation is desflurane > sevoflurane > isoflurane > halothane > TIVA (e.g. propofol).
What is the Treppe (Bowditch) effect?
Increasing the heart rate increases inotropy through the Treppe (or Bowditch) effect?
What is important to note when administering vancomycin?
What do you need to watch out for?
Additionally, administration should not exceed 10mg/min because of the risk of vancomycin infusion syndrome (i.e. “red man syndrome”).
Vancomycin infusion syndrome is characterized by hypotension, tachycardia, generalized pruritus, and red rash.
What is the treatment of methemoglobinemia?
Methylene blue (1-2 mg/kg) is the primary pharmacologic treatment of methemoglobinemia.
If you are in a urology case and you have the choice between indigo carmine and methylene blue, what do you need to check before administering?
Methylene blue is a monoamine oxidase inhibitor. Doses > 5mg/kg in the setting of selective serotonin reuptake inhibitor use may precipitate serotonin crisis.
For this reason, indigo carmine may be preferred for use in detecting ureteral injury.
Why is methylene blue contraindicated in setting of methemoglobinemia with a G6PD patient?
What is the treatment then?
Patients with G6PD-deficiency are unable to generate NADPH sufficiently and therefore methylene blue is not only ineffective, but paradoxically serves as an oxidant stress leading to hemolysis.
Ascorbic acid (vitamin C) can serve as an electron donor (antioxidant), and is the treatment of choice of methemoglobinemia in the setting of G6PD-deficiency.
What is the % collapse of the IVC correlated to in terms of volume?
Collapse <50% suggests volume overload
Caval Index >50% suggests fluid responsiveness
What is the size of the IVC that determines volume depletion vs. overload?
IVC <1.5 cm suggests volume depletion
IVC >2.5 cm suggests volume overload
How does higher altitude affect anesthetic delivery through a vaporizer?
At high altitudes, the partial pressure of desflurane will decrease in proportion to the reduction in atmospheric pressure as it correlates to the calibration pressure, usually 760 mmHg (1 atm)
OR
Another way of saying this is gas delivered will be inversely proportional to altitude
What is dead space broken into?
Anatomic dead space is equal to the volume in the portions of the airways not involved in gas exchange (i.e. oronasopharynx, trachea, bronchi, and most of the bronchioles).
Alveolar dead space is the volume in the alveoli that is not being perfused and not involved in gas exchange.
Physiological Dead Space = The sum of alveolar and anatomic dead space
What is the amount of dead space we have?
What is the dead space to tidal volume ratio?
Approximately 2 mL/kg in the adult patient.
Also, remember that the dead space to tidal volume ratio (Vd/Vt) is usually 33% since tidal volume is approximately 5-7 mL/kg.
How is dead space affected when transitioning from the supine to upright positioning?
Dead space is increased in the upright position relative to the supine position.
This occurs due to an increase in alveolar dead space since the apices of the lungs are essentially non-perfused in the upright position due to the effects of gravity on blood flow distribution.
How does positive pressure ventilation affect dead space?
Positive pressure ventilation (PPV) increases alveolar dead space and can worsen ventilation to perfusion matching.
A spontaneously breathing patient with healthy lungs has a dead space to alveolar ventilation ratio of 1:2 whereas the ratio will be 1:1 with PPV.
Positive intrathoracic pressure can decrease cardiac output and therefore pulmonary perfusion due to decreased venous return to the right heart.
What will large amounts of licorice cause?
Large quantities of natural licorice will induce hyperaldosterone-like effects including:
hypokalemia
hypertension
hypernatremia
Fluid overload
metabolic alkalosis
What is the danger of mixing lidocaine and propofol in the same syringe?
Mixing propofol with lidocaine has been shown to decrease the stability of the propofol emulsion and may cause pulmonary embolism.
The FDA recommends against mixing propofol with any other therapeutic medications prior to administration.
When 40 mg of lidocaine was added, droplets of greater than 5 mcm were detected at 30 minutes. The theoretic human risk of embolism occurs at droplet sizes greater than 5 mcm
What is the 2nd gas effect?
The second gas effect occurs when nitrous oxide and a volatile anesthetic are used concurrently.
Uptake of nitrous oxide is significant because of the high concentration used (the concentration effect).
When a significant amount of nitrous oxide is removed from the alveoli into the blood the relative alveolar concentration of the volatile agent being used increases.
This increase speeds up the anesthetic onset for the volatile agent, which is the second gas effect.
What is the concentration effect?
The concentration effect occurs when a high concentration of anesthetic can be used. Currently the only anesthetic in clinical use in which the concentration effect can be demonstrated is nitrous oxide.
This effect explains why nitrous oxide has a more rapid onset than desflurane.
What is the oil:gas coefficient?
How does this relate to MAC?
Measure of lipophilicity.
The oil:gas coefficient is related to anesthetic potency and minimum alveolar concentration (MAC).
The higher the oil:gas coefficient the lower the MAC.
Isoflurane has an oil:gas coefficient of 99 and a MAC of about 1.
Whereas desflurane has an oil:gas coefficient of 19 and a MAC of about 6.
Rank the solubility of commonly used anesthetics.
Solubility of commonly used anesthetics is:
desflurane > nitrous oxide > sevoflurane > isoflurane from least soluble to most soluble.
(Note des is greater than nitrous)
What nerve is most likely to be injured during brachial artery cannulation?
Medial Nerve

How does a spinal affect GI secretions?
Unopposed parasympathetic stimulation following spinal anesthesia causes increased gastrointestinal secretions.
What is shown here?

After single lung transplantation, the capnogram would be unique for each lung, if they were to be separated.
The transplanted lung, assuming it is healthy, would produce an essentially normal pattern of exhalation (see Figure 1).
The native lung, however, is still diseased and will show an obstructive appearance (slow, blunted upstroke, phase 2–3) and no plateau of exhalation (phase 3-4) is reached.
Combined, this causes a “double peak” appearance, as in Figure 2. The first peak is from the normal (transplanted) lung, while the second peak of the tracing represents exhalation from the native, diseased lung.
What do you do here?
What is this showing?

Replace the Exhausted CO2 absorbent or increase the flows
Capnogram of incompetent inspiratory valve. The expiratory alveolar plateau is extended due to inspiration of CO2-rich gas at the beginning of inspiration. The blue arrows above indicate where inspiration actually began in the patient. The inspiratory phase is shortened, has a blunted downstroke, and the CO2 nadir may or may not reach zero.

What is the capnogram shown here?

Incompentent Expiratory Valve
Garlic, ginger, ginkgo, and vitamin E are all associated with an increased risk of what?
Bleeding
What is the most common risk associated with autologous blood transfusion?
Infection resulting from improper storage is the most common risk associated with autologous blood transfusion.
What are the benefits of autologous transfusion reactions?
- Does not place the recipient at risk of immune-mediated reactions
- Reduce*** the incidence of complications associated with allogeneic donor blood, such as ***hemolytic and non-hemolytic (febrile, urticarial, and post-transfusion purpura) reactions resulting from donor proteins triggering an immune-mediated reaction in the recipient
What is the treatment for central anti-cholinergic syndrome?
Physostigmine is an anticholinesterase with a tertiary amine structure that can be used as a treatment for central anticholinergic syndrome.
Reversal of toxic anticholinergic effects: Note: When administering by IV injection, administer no more rapidly than 1 mg/minute to prevent bradycardia, respiratory distress, and seizures from too rapid administration. Slower administration (ie, over no less than 5 minutes) may be preferable (Howland 2019).
IM, IV: Initial: 0.5 to 2 mg; may repeat every 10 to 30 minutes until response occurs. Subsequent doses may be required to manage life-threatening anticholinergic effects (Krenzelok 2010).
When would Scopalamine IV be recommended?
This is typically done for hemodynamically unstable patients, such as during massive hemorrhage or trauma.
Scopolamine IV may also be given preoperatively to reduce the risk of awareness in selected populations.
Sedation, amnesia: IM, IV, SubQ: 0.3 to 0.6 mg 3 to 4 times/day
What is Poiseuille Law?
The Poiseuille Law states that:
Q = ΔP(π * radius4) / (8 * viscosity * length)
Where: Q = flow, Δ = change in, P = pressure, π = 3.14159… (the mathematical constant).
What are the 3 ways in which you can get cyanide toxicity from Nitroprusside?
Nitroprusside is metabolized to cyanide ions and toxicity can occur following lengthy infusions. The three major mechanisms for nitroprusside toxicity are
1) Cyanide ions bind to cytochrome c oxidase and inhibit cellular aerobic respiration.
2) Formation of cyanmethemoglobin which is unable to carry oxygen.
3) Thiocyanate production which causes CNS-related effects.

What nerve provides sensation to the entire larynx above the glottis? Laryngospasm can result from stimulation of this nerve.
The internal branch of the superior laryngeal nerve (SLN) provides sensation to the entire larynx above the glottis. Laryngospasm can result from stimulation of this nerve.

What are the neurological changes in the elderly?
Comment on:
- Gray/What matter
- Neurotransmitter #
- Epidural and CSF
- Dura Mater Permebility
Nervous System
- Decreased gray/white matter
- Decreased neurotransmitters (acetylcholine, dopamine)
- Decreased epidural space
- Decreased CSF volume
- Increased permeability of dura mater
What are the cardiovascular changes in the elderly?
Comment on:
Ventricular function
Autonomic response
Large artery changes
Afterload
Diastolic Function
LVEDP
Cardiovascular System
- Decreased ventricular compliance (dependent on atrial kick)
- Decreased beta-receptor responsiveness (decreases maximal heart rate)
- Increased sympathetic nervous system activity
- Increased stiffness of large arteries (Hypertension common)
- Increased afterload
Decreased diastolic dysfunction
LVEDP increases (due to decreased diastolic dysfunction)
How does the respiratory system change in the elderly?
Comment on:
VC, RV, CC
Dead space
Lung compliaance
PVR
- *Respiratory System**
- Decreased vital capacity
- Increased residual volume
- Increased closing capacity
- Increased anatomic dead space
- Increased lung compliance
- Increased pulmonary vascular resistance
How does the hepatic blood flow change with age?
Decreases
What is causing this artificat on EKG?

AC power source
Interference from an alternating current (AC) source produces a thick ECG tracing which alternates up-and-down 60 times a second (60 hertz) in the United States, see above.
Interference can occur if an AC power cord is lying across the ECG cable or the ECG monitor. It can also occur if the ECG cable or monitor is too close to an AC source.
How does a right to left shunt affect rate of inhaled induction?
How does a right to left shunt affect rate of IV induction?
A right-to-left intracardiac shunt slows the rate of inhalational induction of anesthesia since the shunted blood is not involved in gas exchange within the alveoli.
Conversely, a right-to-left shunt speeds the rate of intravenous induction because a portion of the drug bypasses the lungs, directly enters the left side of the heart and is quickly delivered to brain tissue. Note, if you feel these sentences contradict each other please re-read carefully.
How does CO affect onset of induction for inhaled agents?
Decreased CO = Faster induction
allows for increased partial pressures of anesthetic gases within the alveoli, resulting in a faster rate of induction. The alveolar partial pressures of the anesthetic remain elevated since less blood can interface with the same number of alveoli filled with a volatile agent. The slow diffusion of a volatile agent into the blood results in faster delivery of the agent to brain tissue. This is because a greater volume of blood is saturated with volatile agent per liter of cardiac output delivered per unit of time.
Higher CO = Slower induction
more blood is involved in gas exchange resulting in increased uptake of anesthetic gas and a larger dilutional effect. This decreases the partial pressure of gas within the alveoli, reduces delivery to the brain, and slows the rate of induction.
How does the volatile agent change the rate of induction?
The effect of cardiac output on the rate of induction is particularly accentuated in agents that have a higher blood:gas partition coefficient. This partition coefficient is a distribution ratio describing how the volatile anesthetic distributes itself between liquid (blood) phase and the gaseous phase (in the alveoli) when the partial pressures are identical in both phases.
If the blood:gas partition coefficient is > 1, then more drug is dissolved in the blood (higher solubility, such as isoflurane or halothane).
If the blood:gas partition coefficient is < 1, more drug exists in the gaseous state and not dissolved in blood (lower solubility, such as desflurane or nitrous oxide). These relationships hold as long as the partial pressures are equal in both phases.
Blood:gas partition coefficients of key anesthetic gases
Desflurane 0.42
Nitrous Oxide 0.46
Sevoflurane 0.69
Isoflurane 1.46
Halothane 2.54
The rate of a rise of FA:FI as a function of cardiac output. A decrease in cardiac output (e.g. from 10 L/min to 2.5 L/min) will increase the alveolar partial pressures of the volatile agent by slowing uptake and speeding the rise of FA:FI ratio. This effect is less pronounced in the insoluble volatile anesthetics (e.g. desflurane) and more pronounced in the soluble ones (e.g. isoflurane). The effect of changing cardiac output is less pronounced for the more insoluble anesthetics.
Why are new generation CO2 absorbents much less likely to react with volatile anesthetics and generate carbon monoxide?
New generation carbon dioxide absorbents do not contain strong bases and as a result, are much less likely to react with volatile anesthetics and generate carbon monoxide.
When should FFP be avoided?
Fresh frozen plasma should be avoided in the following situations:
- Use to correct mildly elevated INR (< 1.8) without signs of bleeding
- Use to correct a vitamin K deficiency that could be corrected with vitamin K
- Use as a primary volume expander (absolute contraindication)
- Use to correct a factor deficiency when recombinant factor replacement is available
What are the effects of Dopamine at low doses?
What receptor?
What is the dose?
Low dosing: 0.5 to 3 mcg/kg/min
At lower concentrations, dopamine primarily acts on the D-1 dopamine receptors to produce vasodilation of the renal, mesenteric, and coronary vasculature.
The main result is an increase in the glomerular filtration rate and renal blood flow (RBF)
Furthermore, low dose dopamine will result in sodium excretion which will increase urine output.
What is the effect of Dopamine at moderate doses?
What receptors?
What effects?
Moderate dosing: 3 to 10 mcg/kg/min
Beta-1 Receptors
- Contractility increases (Ionotropy)
- Minimal change in HR anad SVR
- Increase in splanchnic and renal blood flow

What is the effect of Dopamine at high doses?
What receptors?
What effects?
High dosing: >10 mcg/kg/min
Higher doses will stimulate the Alpha 1 and beta-1 receptors to increase release of norepinephrine from sympathetic nerve terminals.
The end result is an increase in heart rate, systolic blood pressure, and pulse pressure.
Diastolic blood pressure is minimally affected however pulmonary vascular resistance may increase.
At the highest doses, dopamine administration will stimulate alpha-1 receptors which results in peripheral vasoconstriction.

Why is it important to know what cyclopentolate is?
Cyclopentolate is an anticholinergic drug often used during ocular surgery to induce mydriasis that can lead to CNS toxicity, including convulsions, if absorbed systemically.
What are the 5 receptors in the chemoreceptor trigger zone and why is this important?
The area postrema of the brainstem, also known as the chemoreceptor trigger zone, contains dopamine, serotonin, acetylcholine, histamine, and neurokinin type 1 receptors.
Dopamine - Haloperidol
Serotonin - Zofran
Acetylcholine - Diphenhydramine, Scopalamine, other Anti-cholinergics
Histamine - Diphenhydramine
Neurokinin 1 - Aprepitant
Antagonism of these receptors can help treat and prevent nausea. However, any medication leading to central antagonism of dopamine receptors can potentially cause EPS.
What are the reasons for using or not using cricoid pressure?
Although routinely used to decrease risk for aspiration, recent evidence suggests cricoid pressure may actually increase the risk for reflux in anesthetized patients.
Cricoid pressure decreases LES tone without influencing gastric pressure.
This leads to a decreased barrier pressure and therefore potentially increases the risk of aspiration.
The clinical significance of this, however, has not been determined.
Opponents of cricoid pressure affirm that succinylcholine increases LES tone, making the need for cricoid pressure during rapid sequence induction (RSI) questionable.
Cricoid pressure continues, however, to be the standard of care during RSI.
What is the duration of action of midazolam compared to flumaazenil?
The duration of action of midazolam (half-life 1.7-2.6 hours) exceeds that of flumazenil (half-life 0.7-1.3 hours). This makes recrudescence of benzodiazepine-induced somnolence after metabolism of flumazenil likely in this context.
Approximately half
What is efficacy vs. potency?
Which is dose dependent?
Efficacy is the maximum effect of a drug. It does not depend on dose.
Potency is the relative dose required to achieve a given effect and is related to receptor affinity and is dose dependent

For partial agonists, what is their relationship of their potency* and *efficacy in comparison to full agonists.
The potency of a partial agonist may be higher than that of a full agonist.
The efficacy of a partial agonist cannot be higher than that of a full agonist.
How long does sodium bicitra take to work? (Onset)
How does it affect gastric pH and volume?
Antacids such as 0.3 M sodium bicitra can be given 15-30 minutes prior to induction to increase gastric pH, however, they will increase gastric volume which is not of particular concern as the real damage to the lungs with aspiration will be caused by the acidity of the fluid, not necessarily the volume.
H2 blockers
Onset?
Duration of action?
Effect on pH and Gastric fluid?
decrease the secretion of gastric acid, resulting in a reduction in gastric volume and an increase in gastric pH. They can be used for aspiration prophylaxis in at-risk patients.
H2 blockers must be given approximately 30 minutes prior to induction of anesthesia to have effectiveness and they last for approximately 9-12 hours (except cimetidine which only lasts 3-4 hours and has multiple side effects such as P450 inhibition, hypotension, arrhythmias and CNS depression). They have no effect on gastric fluid already present and do not speed clearance of gastric fluid already present.
How does Metoclopromide work?
Effect on pH and gastric volume?
Onset?
Metoclopramide is a gastrokinetic drug that relaxes the pylorus and duodenum and increases upper GI motility to increase clearance of gastric fluid, however, it does not alter gastric fluid pH.
Metoclopramide also increases lower esophageal sphincter tone because of cholinomimetic actions and can have extrapyramidal side effects as it is a D2 receptor blocker.
What do you need to remember about IgA deficiency with other blood products besides PRBC?
Since plasma cannot be washed of IgA, it must come from a deficient donor.
What is activated during a hemolytic transfusion reaction?
What end organ damage can happen?
What antibody mediated response can happen and why?
The complement system is activated in a hemolytic transfusion reaction that typically occurs when ABO incompatible blood is given.
A hemolytic transfusion reaction will result in the destruction of donor red blood cells and the release of hemoglobin in the blood.
Free circulating hemoglobin can precipitate in the kidneys resulting in kidney failure.
The antibody mediated immune response results in the release of bradykinin, serotonin, and histamine leading to flushing, bronchospasm, and hypotension.
In addition, 30-50% may develop disseminated intravascular coagulation (DIC).
If the patient is under general anesthesia, initial signs include hypotension and hemoglobinuria.
A patient has a history of non-hemolytic transfusion reaction. What is the recommendation for the next surgery that requires blood products?
Nonhemolytic
Caused by cytokines and the release of intracellular contents in leukocytes.
In addition to fever, patients may have chills, myalgias, headache, and non-productive cough.
The use of leukoreduced blood minimizes this reaction.
What happens during graft vs. host disease?
What measure should you take if you have a GVHD patient with anticipated blood products?
Graft versus host disease may occur in immunocompromised patients when viable donor lymphocytes become engrafted, proliferate, and establish an immune response against the recipient. Other patients at risk are individuals receiving blood from a close relative.
The host does not recognize the lymphocytes as foreign while the donor cells recognize the host as foreign.
Measure = Blood given to immunocompromised patients and blood from first degree relatives should be irradiated to inactivate donor lymphocytes.
You have a patient with cirrhosis.
How is the NMBD dose affected?
Intubating doses of neuromuscular blocking drugs are increased in cirrhosis because of the increase in volume of distribution.
Maintenance doses of hepatically metabolized and/or cleared drugs should be reduced and neuromuscular function carefully monitored as duration of action is prolonged.
**Paradox**
Neuromuscular blocking agents are highly water-soluble due to quaternary amine structures and thus an increase in body water, such as with cirrhosis, CHF, or renal failure, results in decreased plasma concentration of neuromuscular blockers requiring an increase in intubating dose.
It is important to remember, however, that clearance of drugs dependent on hepatic metabolism is reduced and therefore they will have a prolonged duration of action. Rocuronium, vecuronium, and pancuronium will all have increased duration of action secondary to impaired hepatic metabolism (caveat: rocuronium is not metabolized but is cleared by the liver in the bile) and therefore maintenance dosing should be reduced and neuromuscular monitoring carefully followed.
What is the breakdown product of Cisatracurium?
Why is this clinically relevant vs not clinically relevant?
What class of NMBD is this?
A breakdown product, laudanosine, is cleared by the liver but levels produced are insignificant clinically.
Laudanosine decreases the seizure threshold, and thus it can induce seizures if present at sufficient threshold concentrations; however such concentrations are unlikely to be produced consequent to chemodegradable metabolism of clinically administered doses of cisatracurium or atracurium..
Laudanosine is a benzyltetrahydroisoquinoline alkaloid
How is the duration of succinylcholine affected by severe end stage liver disease?
apnea time is increased from 3 minutes to 9 minutes.
What affects a local anesthetics potency?
1) Anesthetic potency = lipid solubility
- Higher solubility enhances diffusion through nerve sheaths/membranes.
- Less lipid solubility results in less potent agents (Less diffusion)
(ex: procaine is the least lipid-soluble and least potent LA, bupivacaine is highly lipid soluble and the most potent.
What affects a local anesthetics onset of action time?
2) Time to onset/Onset of action = pKa = dissociation constant: determines the pH at which a drug’s ionized (charged) and unionized (uncharged) forms are in equal concentrations.
This determines the amount of drug in the unionized form. This is why many local anesthetics do not work in acidic areas such as abscesses.
pKa Lidocaine = 7.9
pKa Bupivicaine = 8.1
pKa Chloroprocaine = 9 (Paradox due to volume)
What affects a local anesthetics duration of action?
Duration of action = protein binding.
- Agents that are highly protein bound will remain bound for longer, increasing the duration of action.
- Duration of action is also influenced by peripheral vascular effects (such as use of vasoconstrictors)
* Why we add epinephrine to our local anesthetics*
What affects local anesthetic absorption?
(Think about route of administration)
ICEBALLS
Location of injection = some areas of the body will have more local anesthetic absorbed. Mnemonic: BICEPSS - Intravenous (Blood) > Intercostal > Caudal > Epidural > brachial Plexus > Axillary > (lower lumbar) Sciatic > Subcutaneous
What is the max dose of procaine?
Length of action?
Procaine
7 mg/kg
15-60 minutes
Chloroprocaine max dose (With and without epi)?
Duration of action?
Chloroprocaine
Max = **11 - 14 mg/kg** (Without vs. with epinephrine) Duration = **15-30 minutes**
How long does Lidocaine last with epi?
Without epi?
- *30-60** without epi
- *120-360** with epi
What is the max dose of mepivacaine?
mepivacaine
Max = 7 mg/kg
Duration:
- 45-90 without epi
- 120-360 with epi
Bupivacaine:
Max dose with and without epi?
Duration of action with and without epi?
Bupivacaine
Max dose
- 5 (not to exceed 175mg or 70cc)
- 5 (not to exceed 225mg or 90cc)
Duration
120-140 without epi
180-240 with epi
What are the 3 factors that affect height of an intrathecal block?
Factors affecting the height of an intrathecal block include:
- Patient position
- Baricity of anesthetic
- Dose of local anesthetic

What are some scenarios where bradycardia is more common following spinal (Intrathecal) anesthetic?
Bradycardia following spinal anesthetic injection is more common in patients with:
1. High baseline vagal tone
2. Anesthetic levels above T5
3. Associated with decreased cardiac preload (i.e. reverse Bainbridge reflex).
Why can bradycardia happen after a spinal?
- Sympathectomy of cardioaccelatory fibers (T1 - T4)
- Drop in preload and SVR (decreased stretch on myocardium which will result in “reversed bainbridge reflex”)
- High Baseline vagal tone (Young atheletes, vasovagal syndromes or autonomic nerve pathology)
- Decreased mechanoreceptor stretch can induce the Bezold-Jarisch reflex with increased parasympathetic output, contributing to bradycardia.
Normally, the carotid sinus baroreceptor response to hypotension (increased heart rate and contractility) dominates, but in the setting of an acute drop in ventricular preload (e.g. spinal anesthetic or nitrate use), the Bezold-Jarisch reflex may occur before the carotid sinus reflex.
For TEG, what is the graph look like?
What are the 4 criteria you look for?
Thromboelastography (TEG) is both a quantitative and graphic evaluation of clotting function. Typical management is:
R value
K value
Alpha Angle
MA value

Thromboelastography (TEG) MA value decreased.
What is the treatment?
MA value decreased -> platelets.
Thromboelastography (TEG) K value prolonged
What is the treatment?
Cryoprecipitate
Thromboelastography (TEG) R value prolonged
What is the treatment?
FFP
If you have a teardrop configuration on TEG (Thromboelastography), what is the treatment?
Anti-fibrinolytics (TXA)
For TEG, what is the “R” from a pathophysiologic perspective?
What is the width (Amplitude)?
What is the normal range?
Reaction time (R) is from time zero to initial clot formation, defined as a width (amplitude) of 2 mm. Normal range is 1-3 minutes.
For TEG, what is the “K” time? What does this mean physiologically?
What is the amplitude range?
If prolonged, how do we treat this
Coagulation time (K) measures speed of clot formation and strengthening. It is equal to the time from amplitude of 2 mm to 20 mm and relies on fibrinogen.
Treatment = Cryoprecipitate
For TEG, what is MA?
what does this reflect?
What is the normal range?
Maximum amplitude (MA), measures the strength of the fully formed clot. It is the maximal width of the TEG. This reflects clot strength as determined by platelet number and function (primarily) as well as fibrin cross linking. Normal is 50-60 mm.
What is the alpha angle on TEG represent?
What is normal value?
What does this rely on?
Alpha-angle is the speed of clot formation, and is represented by the angle between baseline and a line tangential to the TEG at 2 mm amplitude. Like the K value, this relies on _fi_brinogen. Normal alpha angle is 45-55 degrees.
What class of medicine is echothiophate?
What is the clinical consequences of this drug?
Echothiophate is an anticholinesterase used to treat refractory glaucoma by causing miosis. Systemic absorption can cause up to a 95% decrease in plasma butyrylcholinesterase (pseudocholinesterase) function. Patients using echothiophate eye drops have demonstrated prolonged neuromuscular block after succinylcholine administration. It may take up to 4-6 weeks after discontinuation of the drug for normal butyrylcholinesterase activity to return.
What are the 3 things that stimulate renin release?
Renin secretion can be caused by 3 different mechanisms:
- Decrease in serum NaCl concentration sensed by the kidneys
- Decrease in blood pressure sensed by kidney baroreceptors
- Activation of renal B1 receptors by norepinephrine.
What are the effects of Angiotensin II on:
BP?
Vascular Smooth Muscle?
Autonomic nervous system?
Thirst?
CV system?
Renal system?
Hormones?
Increase in blood pressure caused by an increase in systemic vascular resistance
Activates the angiotensin1 (AT1) receptors on vascular smooth muscle cells, causing constriction of the arterioles
Augments the sympathetic nervous system by inhibiting norepinephrine reuptake into nerve terminals. It also stimulates the release of catecholamines from the adrenal medulla.
Centrally mediated increased thirst and enhances the release of vasopressin. These effects are almost immediate, but are not long-lasting.
Stimulates remodeling of the cardiovascular system, causing hypertrophy of cardiac cells and increased collagen deposition
Decrease in the urinary excretion of both sodium and water.
Stimulates the adrenal cortex to synthesize and release aldosterone.
Aldosterone also acts on the kidneys to decrease the urinary excretion of sodium and water. This ultimately leads to an increase in blood volume and therefore blood pressure.
What is the Pros and Cons of using opiates in an epidural compared to Local Anesthetics alone?
Pros
Use of opioids as adjuncts for neuraxial anesthesia and analgesia can significantly enhance block quality, density, and duration of analgesia.
Cons
However, this comes at the price of an increased risk of side effects including sedation, respiratory depression, pruritus, and nausea/vomiting, compared to local anesthetics alone.
What is the recommended monitoring time for epidural morphine vs. epidural fentanyl?
The ASA recommends a longer period of monitoring for respiratory depression following a single neuraxial dose of morphine compared to fentanyl.
For lipophilic opioids: continual monitoring for at least 20 minutes following administration and then at least hourly monitoring for the next two hours.
For hydrophilic opioids: at least hourly monitoring for the first 12 hours and then monitoring at least every two hours for the next 12 hours.
What is a mnemonic to help recall light absorption by wavelength?
A mnemonic to help recall light absorption by wavelength is SeXy DARLing: at SiX hundred wavelength, Deoxyhemoglobin Absorbs Red Light.
What nm is red light at?
What nm is infrared light at?
At red wavelengths, what type of hemoglobin absorbs more light?
At infrared wavelengths, what type of hemoglobin absorbs more light?
Red light at 660 nm and infrared light at 940 nm (some manufacturers use 905, 910, or 920 nm) is pulsed through the tissue. A ratio of the absorbance of red to infrared light is calculated and that calculated number is translated to the oxygen saturation reading.
At red wavelengths, deoxyhemoglobin absorbs more light than oxyhemoglobin
At infrared wavelengths, oxyhemoglobin absorbs more.
What is the conversion factor for determining height difference correlated to blood pressure?
(Include in here a mneumonic to help remember this)
The difference in blood pressure at 2 different sites equals the height difference in cm multiplied times the conversion factor 0.74.
Ex: height difference of 22 cm, equaling 16
A mnemonic to help remember which comes first (pressure or height) is “pH” or “pH 15 20”, where a pressure of 15 mmHg correlates to a height of 20 cm.
What arae some triggers to Masseter Muscle Spasm?
Several risks for MMR have been proposed and include:
- Inadequate dose of succinylcholine
- Inadequate time for onset
- Side effect of other induction medications (etomidate, high dose synthetic opioids)
- Temporomandibular joint dysfunction
- Duchenne muscular dystrophy
- Myotonia congenita, and possibly other muscle disorders.
Fresh gas flow (FGF) must be greater than or equal to “X” times minute ventilation in the Mapleson D semi-open breathing system to prevent rebreathing during controlled ventilation.
The FGF during spontaneous ventilation must be equal to “X” times minute ventilation in the Mapleson D, E, and F circuits
Fresh gas flow (FGF) must be greater than or equal to 1-2 times minute ventilation in the Mapleson D semi-open breathing system to prevent rebreathing during controlled ventilation.
The FGF during spontaneous ventilation must be equal to 2-3 times minute ventilation in the Mapleson D, E, and F circuits.
What are the 4 advantages of a Bain circuit (coaxial version of a Mapleson D system)?
The advantages of a Bain circuit (coaxial version of a Mapleson D system) include:
(1) conservation of moisture due to partial rebreathing
(2) warming of fresh gas inflow by surrounding expiratory gas
(3) scavenging waste is easier from the APL valve
(4) advantageous when access to the patient is limited.

How does metabolism of drugs work (for most drugs)?
Most drugs occurs via two processes, phase I and phase II metabolism.
Phase I includes oxidative and reductive reactions including hydrolytic reactions and those catalyzed by the hepatic cytochrome P450 enzymes
Phase II reactions involve conjugation of the drug or its metabolite(s) with a substrate such as glucuronic acid. Once a drug is metabolized, it is much more efficiently eliminated from the body.
Per ASA guidelines, what are the 3 aspects of monitoring circulation that must be maintained through an anesthetic?
The patient’s circulatory function must be continually monitored via three mechanisms.
- First, continuous ECG must be used from the beginning of anesthesia until preparing to leave the anesthetizing location.
- Second, arterial blood pressure and heart rate must be determined and evaluated at least every five minutes.
- Finally, pulse monitoring must be continually evaluated such as via pulse palpation, heart sound auscultation, intraarterial pressure monitoring, and/or pulse plethysmography or oximetry.
What is the ASA Guidelines when determining if temperature monitoring should be required?
In all anesthetics in which “clinically significant changes in body temperature are intended, anticipated, or suspected” temperature must be monitored.
What is the % of Intracellular vs extracellular volume of total body water?
What is the ECV divded into? (include percentages)
Intracellular volume (ICV) - The ICV contains two-thirds of TBW and represents 40% of total body weight.
Extracellular volume (ECV). The ECV contains one-third of TBW and represents 20% of total body weight.
Subdivided into plasma volume and interstitial fluid volume.
Plasma volume comprises 20-25% of the ECV and represents 4% of total body weight.
Interstitial fluid volume comprises 75-80% of the ECV and represents 16% of total body weight.

What is the incidence of bradycardia with sugammadex?
Bradycardia may occur in patients treated with sugammadex.
The incidence is approximately 1% with 2 mg/kg and 4 mg/kg doses, and 5% with 16 mg/kg doses.
How does intrapulmonary shunt affect the speed of inhalational induction?
Intrapulmonary shunt reduces the speed of inhalational induction.
When determining V/Q mismatches, when is the speed of induction increased vs. decreased?
When perfusion is affected (reduced cardiac output, pulmonary embolism), the speed of induction of inhalational agents is increased. The impact is more significant with soluble agents such as isoflurane. The rise of FA/FI is steeper with lower perfusion of the lungs.
When ventilation is affected (intrapulmonary shunt), the speed of induction of inhalational agents is decreased. This occurs to a greater degree with insoluble agents (desflurane, sevoflurane). The rise of FA/FI is less steep.
Who should receive prophylaxis for infective endocarditis?
(Hint: 6 high risk conditions that then need to have what 3 types of surgeries?)
- *The list of patients who should receive prophylaxis is below:**
- Patients with a prosthetic cardiac valve
- Patients who have history of infective endocarditis
- Patients with unrepaired cyanotic congenital heart disease (including palliative shunts/conduits)
- Patients with congenital heart defects which were repaired with prosthetic material within 6 months of the procedure
- Patients with repaired congenital heart disease with residual defects at the site, or adjacent to the site, of a prosthetic patch or device
- Cardiac transplantation recipients who develop cardiac valvulopathy (substantial leaflet pathology and regurgitation)
- *Of note, prophylaxis only applies to certain surgical procedures:**
- Dental* procedures that involve *manipulation of gingival tissue or the periapical region of teeth or perforation of the oral mucosa
- Invasive respiratory tract* procedures that involve *incision or biopsy of the respiratory mucosa (e.g. tonsillectomy, adenoidectomy)
- Infected skin, skin structure, or musculoskeletal tissue
What is the recommended Rx for infective endocarditis prophylaxis?
The preferred prophylactic regimen is Amoxicillin 2 g given as a one time oral dose 30-60 min before the procedure.
Explain the physiology behind negative pressure pulmonary edema and how it develops?
This negative force can be upwards of -50 to -100 cm H2O of pressure.
The negative pressure causes a significant increase in preload, thereby increasing pulmonary blood volume. There is also a significant increase in left ventricular afterload, which causes a decreased cardiac output.
The increase in pulmonary blood volume along with a decrease in cardiac output will increase the pulmonary transudative pressures. With all this occurring, pulmonary vascular resistance increases causing a shift of the intraventricular septum. The ventricular septal shift to the left causes a left ventricular diastolic dysfunction, which further increases pulmonary hydrostatic pressures.

What are the 4 active cardiac conditions that require further workup before elective surgery?
Active cardiac conditions mandate further preoperative evaluation and treatment.
- *Active Cardiac Conditions:**
1) Unstable coronary syndromes
(UA, MI)
2) Decompensated heart failure
(NYHA class III or IV, severe angina) 3) *_Significant arrhythmias_*
(high-grade AV block, Mobitz type II AV block, third-degree AV block, symptomatic ventricular arrhythmias, supraventricular arrhythmias (including atrial fibrillation) with uncontrolled ventricular rate > 100 bpm at rest, symptomatic bradycardia, and newly recognized ventricular tachycardia)
4) Severe valvular disease
Severe aortic stenosis (mean pressure gradient > 40 mmHg, aortic valve < 1.0 cm^2, or symptomatic) and symptomatic mitral stenosis (progressive dyspnea on exertion, exertional presyncope, or heart failure).
What are the 6 revised cardiac risk index variables?
- *Revised Cardiac Risk Index Variables:**
1) History of ischemic heart disease
2) History of congestive heart failure
3) History of cerebrovascular disease
4) Insulin therapy for diabetes
5) Preoperative serum creatinine > 2.0 mg/dL
6) High-risk type of surgery
What is the 4 criteria needed to diagnose TRALI (Transfusion related acute lung injury)?
Criteria:
1) Acute lung injury (ALI)
- Acute onset
- Hypoxemia (PaO2:FiO2 ≤ 300 mm Hg or SpO2 < 90%
on room air, or other clinical evidence of hypoxemia
- Bilateral infiltrates on frontal chest radiograph
- No evidence of left atrial hypertension as the sole
explanation for the clinical findings
2) No pre-existing ALI before transfusion
3) Onset during or within 6 hours of transfusion
4) No temporal relationship to an alternative
risk factor for ALI
TACO requires 3 or more of which of the following criteria within 6 hours of transfusion?
New onset or exacerbation of three or more of the following within 6 hours of transfusion:
- Acute respiratory distress (dyspnea, cough, orthopnea) Symptoms
- Increased brain natriuretic peptide (BNP) Labs
- Increased central venous pressure (CVP) Hemodynamics
- Evidence of left heart failure Clinical diganosis / Echo
- Evidence of positive fluid balance I/O balance
- Radiographic evidence of pulmonary edema Imaging
When you hear, Left shift vs. Right shift on the Hemoglobin dissociation curve, what is the x and y axis?
Y axis = % Hemoglobin Saturated (SaO2)
X axis = PaO2

How does MAC change with age?
Excluding patients less than one year of age, the MAC of each of the potent anesthetic gases declines with age.
There is a linear model that describes the change in MAC of approximately 6% per decade, a 22% decrease in MAC from age 40 to age 80, and a 27% decrease in MAC from age 1 to age 40 years.
How does MAC requirements change up to the age of 1?
Which volatile anesthetic has the highest MAC requirement?
MAC rises at one month of age, peaks at approximately 6 months of age, and regresses back to normal at 1 year of age.
Sevoflurane demonstrates a much higher increase in MAC compared to the slight increase with desflurane and isoflurane.
The MAC of sevoflurane is often up to 2.5-3% higher in patients 1 month to 1-year-old.
How does puberty affect MAC requirements?
Increases MAC Requirements
What is the mechanism for tetanus?
Tetanus acts by preventing neurotransmitter release (glycine and GABA) from inhibitory neurons in the spinal cord.
The lack of inhibition causes increased muscle contractions to the point of tetanus.

What is the mechanism for botulism?
Botulism toxin has a similar mechanism of preventing neurotransmitter release (acetylcholine), but botulism affects the alpha motor neuron causing flaccid paralysis.

What are the three classic “Carcinoid triad” S/S?
What is carcinoid syndrome cause from?
The classic “carcinoid triad” consists of flushing, diarrhea, and right-sided cardiac involvement.
It is caused by tumor release of vasoactive amines* (e.g. serotonin, norepinephrine, histamine, and dopamine), *polypeptides* (e.g. bradykinin, somatostatin, vasoactive intestinal peptide, and glucagon), and *prostaglandins.
The cardiac involvement is generally limited to only the right side of the heart since, as discussed above, most of the vasoactive hormones are metabolized while in the pulmonary circulation before returning to the left side of the heart.
How do calcium channel blockers affect Neuromuscular blocking drugs?
KEY = Acute vs. Chronic CCB usage
Calcium channel blockers can augment depolarizing and nondepolarizing muscle relaxants, particularly when the two drugs are administered concurrently to CCB-naïve patients (Calcium channel blockers given acutely)
Consider the careful titration of NBDs in this setting. In patients taking chronic CCBs, the augmentation effect is clinically insignificant.
Cytochrome p450 is involved in what phase of metabolism by what reaction to drugs?
Cytochrome p450 is involved in phase I metabolism by oxidizing many drugs.
What is the equation used for Maximum Allowable Blood Loss?
(Hint: List the estimated blood volume for each age group)
The MABL of a patient = [EBV * (starting Hct - target Hct)] / (starting Hct).
Age Group. EBV (mL/kg)
Premature infant 90-105
Full term newborn 80-90
Infant (3 months - 1 year) 70-80
Child 1-12 years 70-75
Adult Female 60-65
Adult Male 65-70
Local Anesthetic allergies are incredibly rare.
If a patient has antigenic reaction to amide reaction, what could be the culprit?
Methylparaben is the methyl ester of para-hydroxybenzoic acid, which is structurally similar to para-aminobenzoic acid (PABA), differing only in having a hydroxyl group vs amino group in the para position.
Thus, cross-sensitivity between these compounds can occur.
If a patient has an antigenic reaction to epinephrine, what could be the culprit?
Sulfites such as sodium metabisulfite are added as antioxidants for the catecholamine content.
Lidocaine vials with epinephrine will typically contain metabisulfite, which may also elicit allergic responses.
You are in the cardiac room undergood deep hypothermic arrest.
How does pH, PaCO2 and PaO2 change with a decrease in temperature?
pH: increases 0.017 per °C decrease
PaCO2: decreases* 2 mm Hg per °C *decrease
PaO2: decreases* 5 mm Hg per °C *decrease
Blood pH and the solubilities of gases in blood are inversely related to temperature while the partial pressures of gases are directly related to temperature.
What is the class, dose and potential side effects of promethazine used for PONV?
Class: Antihistamine (H1 antagonist used as an antiemetic)
Dosing: Nausea and vomiting: IV, rectal: 12.5 to 25 mg every 4 to 6 hours, as needed
Administration: Through non-patient IV can cause tissue necrosis
IV use should be avoided when possible since severe tissue damage has occurred with IV administration; in selected patients, promethazine has been diluted and infused at a maximum rate of 25 mg/minute.
To minimize phlebitis, consider administering over 10 to 15 minutes, limiting initial dose to 1/4 or 1/2 the usual dose (eg, in adults 6.25 to 12.5 mg), further diluting the 25 mg/mL strength in 10 to 20 mL NS, and administering through a large bore vein (not hand or wrist) (Reynolds 2014) or via a running IV line at port farthest from patient’s vein
How can you remember how height affects mmHg changes with BP?
Ex: Sitting beach chair blood pressure measurements
A mnemonic to help is “pH 15 20” to remember the pressure of 15 mm Hg is equal to a change in height of 20 cm.

When evaluating a post operative ulnar neuropathy what are the paths to determine a diagnosis?
Nerve conduction studies are beneficial in evaluating both motor and sensory deficits.
Evaluate between existing subclinical polyneuropathy and some distinction of axon loss and demyelination
Electromyography can help determine the timing of the nerve injury.
For ulnar neuropathy, what are the risk factors?
When does it present?
- Male
- Thin or Obese (BMI > 38)
- Prolonged bed rest
- Genetic predispositon (Bilateral neuropathy found)
Presents 48 hours after surgery
You have a patient who has a sensory neuropathy vs. motor neuropathy. What do you tell them?
Sensory = Resolve in 5 days (self limiting)
Motor = Neurology consult
Always seek neurologic consultation. Sensory injuries usually resolve, whereas motor injuries are more severe and mandate EMG (can help determine if it was present preoperatively, as denervation signs take ~ 3 weeks to develop).
Reversible motor nerve injuries usually take 3-12 months to recover and require physical therapy to prevent atrophy and contractures in the interim
What is the relationship between pKa and pH for local anesthetics?
Local anesthetics with a pKa closer to a physiologic pH will have more drug in the nonionized form, and therefore more drug available for use.
pH < pKa = ionized.
pH = pKa, 50% of the molecules will be ionized and 50% will be unionized.
pH > pKa = Non-ionized
What is the relationship of CBF to:
PaO2?
Temperature
PaCO2?
MAP?
ICP?
Cerebral blood flow is:
Inversely related to PaO2 when less than 50 mm Hg while remaining unchanged with PaO2 > 50 mmHg
Directly related to body temperature
PaCO2 (within normal physiologic ranges)
Extremes of MAPs (< 50 or >150 mm Hg) and remains unchanged within the autoregulatory range of MAPs (50-150 mm Hg)
ICP

Regarding local anesthetics, what is the order of blockade?
Motor Blockade
Sensory / Pain
Sympathetics
Differential blockade with local anesthetics results in sympathetic blockade first, followed by pain/sensory blockade, then motor blockade last
List the different susceptibilities of:
A-alpha
A-Beta
A-Delta
A-Gammy
C fibers
This is (at least in part) explained by differential susceptibility of nerve fibers to local anesthetics being:
A-delta, A-gamma > lA-alpha, A-beta > C.
What are the equations for compliance and elastance**, respectively?
Compliance = Change in Volume / Change in Pressure
Elastance = Change in Pressure / Change in Volume
What is the equation for Static Respiratory Compliance?
What is the equation for Dynamic Respiratory Compliance?
Remember, Compliance is [Change in Volume] / [Change in Pressure]
calculate static respiratory system compliance:
CS = [Tidal Volume] ÷ (Plateau Pressure – PEEP)
Dynamic = Just replace the plateau pressure with peak pressure during inspiration
The gauge pressure on Nitrous Oxide tank will continue to read what psig (“full”) until approximately what percent % or how many liters of the nitrous oxide remains?
The gauge pressure will continue to read 745 psig (“full”) until approximately 16% or 253 liters of the nitrous oxide remains.
If a nitrous oxide tank weights 6.5 kg, how much Volume remains in the tank?
A full E-cylinder of nitrous oxide is approximately 8.80 kg; an empty cylinder is approximately 5.90 kg. At room temperature, one gram of nitrous oxide is equivalent to 0.55 liters of gas at 20 °C. Thus, to calculate the volume of nitrous oxide in an E-cylinder based on weight, the following formula may be used:
Volume remaining (L) = (cylinder weight (g) – 5900 g) x 0.55 L/g
Volume = ( 6.5 - 5.9 ) x 0.55 = 0.33 liters
A nitrous oxide tank shows a gauge pressure of 400 psig.
How much volume remains in the tank?
Volume remaining (L) = (gauge pressure (psig) / 745 psig) x 253 L
Volume = (400/745) x 253 liters = 135 Liters
What are the 4 criteria for diagnosing ARDS?
- Acute onset (within 72 hours)
- Bilateral pulmonary infiltrates on chest radiograph
- Noncardiogenic pulmonary edema
- PaO2/FiO2 ratio of < 300
(The Berlin definition stratifies ARDS into mild, moderate, and severe based on the PaO2/FiO2 ratio of 200-300, 100-200, and < 100 respectively).
What are the ways you could improve oxygenation in Severe ARDS due to true shunt?
application of PEEP, conversion to extracorporeal membrane oxygenation (ECMO), use of inhaled nitric oxide, or inverse ratio ventilation.
Oxygen therapy is of limited value* with *large intrapulmonary shunts due to limitation of gas exchange and requires alternative therapies to improve V/Q matching.
What is the difference between shunt vs. dead space?
V/Q
Dead space = Ventilation but no blood flow
Shunt = No ventilation but blood flow

Non-Anion Gap Metabolic Acidosis (NAGMA)
3 main causes?
Nonanion gap metabolic acidosis (where the anion gap is normal at 8-12 mEq/L) is typically caused by
1. Chloride-containing acid administration
2. Increased HCO3- loss or loss of HCO3- precursors
3. Decreased renal acid excretion
Other than NaCL massive resuscitation, what is a cause of NAGMA (non-anion gap metabolic acidosis) seen?
Total parenteral nutrition often contains ammonium chloride and metabolism of the amino acids it contains can create HCl.
Together, hyperalimentation can lead to excess Cl- and therefore a nonanion gap acidosis.
What are some NAGMA (non-anion gap metabolic acidosis causes of HCO3 loss?
Increased HCO3- loss is usually via the gastrointestinal (GI) tract or the kidneys.
Causes of increased GI losses include diarrhea, GI fistulas (e.g. pancreatic, biliary, bowel), high ostomy output, or use of bile or phosphorus binding drugs.
Renal losses can be caused by use of carbonic anhydrase inhibitors (e.g. acetazolamide) or type 2 (proximal) renal tubular acidosis (RTA).
What are some NAGMA (non-anion gap metabolic acidosis causes decreased acid excretion?
Decreased acid excretion is due to:
- Hypoaldosteronism
- Renal causes including acute and/or chronic renal failure or type 1 (distal) RTA.
What are the two acronyms that can be used for NAGMA (non-anion gap metabolic acidosis) causes?
ACCRUED: Acid infusion, Compensation for respiratory alkalosis, Carbonic anhydrase inhibitors, Renal tubular acidosis, Ureteral diversion, Extra alimentation, Diarrhea.
FUSEDCARS: Fistula, Ureteroenterostomy, Saline administration, Endocrine (hyperparathyroidism), Diarrhea, Carbonic anhydrase inhibitors, Ammonium chloride, Renal tubular acidosis, Spironolactone.
Simplistically, what causes a high anion gap metabolic acidosis?
In patients with a high anion gap metabolic acidosis, HCO3- concentrations decrease as it is consumed buffering a large amount of pathologic anions. This leads to a high anion gap.

What is the mechanism of action of glucagon?
Glucagon also helps raise blood sugar by three indirect effects.
First, it stimulates lipolysis which produces two substrates for gluconeogenesis: glycerol and free fatty acids.
Second, it inhibits hepatic glycolysis. Since glycolysis consumes glucose to create ATP, stopping this process allows glycolytic intermediates to be shunted into the gluconeogenesis pathway.
Third, glucagon inhibits hepatic glycogen synthesis so that available glucose is released into the bloodstream and not stored within the liver.
What effect is seen with paralytics with MS patients?
Depolarizing = Avoid Succinylcholine because patients have denervation or misuse myopathy leading to a higher risk for hyperkalemia
Nondepolarizing agents (Roc, Cis, Vec) can be variable, with a resistance being seen in patients with up-regulation of acetylcholine receptors or a sensitivity in patients with muscle weakness and decreased muscle mass.
How can you simplify use of paralytics in patients with neuromuscular disorders?
Key = think of the disorders as conditions that either up-regulate or down-regulate the number of acetylcholine receptors and then infer what use of a specific type of muscle relaxant will do based on the pathophysiology.
Up-regulation of acetylcholine receptors include multiple sclerosis, burns, stroke, spinal cord injury, Guillain-Barre syndrome, prolonged immobility, and muscular dystrophies.
- Succ = Contra
- Have to use NMBD
Down-regulation of acetylcholine receptors is myasthenia gravis.
- Less susceptible to SCh, more susceptible to non-depolarizing NMBDs
What is the timing of burned/stroke/spinal cord injury patients when using or not using succinylcholine?
Burns (24 hours until 1-2 years after)
In patients who have suffered a burn injury, the Ach receptors typically start to increase after 48-72 hours.
In general, succinylcholine should be avoided after 24-48 hours as fatal hyperkalemia may ensue.
When does prolonged immobility increase risk of reaction of using succinylcholine?
Risk of reaction increases significantly after 16 days
For MS patients, how do you adjust your dose of NMBD?
How is twitch monitoring affected?
If using NDMR give small doses and monitor neuromuscular function: twitch monitor may not be accurate depending on site (orbicularis occuli underestimates muscle paralysis)
What are the anesthetic paralytic implications of Lambert Eaton?
Anesthetic Implications:
Sensitive to Succinylcholine
Sensitive to Non-Depolarizing Neuromuscular Blockers
Poor response to anti-cholinesterases = Neostigmine is ineffective for reversal of NDMR
What are the anesthetic implications of anticholinergic poisoning?
Downregulation of ACh Receptors
- Prolonged response to depolarizing paralytic (Succinylcholine)
Succinylcholine should be avoided as it is degraded by plasma cholinesterase - may result in prolonged paralysis: neostigmine should be avoided since it will worsen the primary problem
What is the management steps for loss of pipeline pressure and/or pipeline crossover?
The management steps for loss of pipeline pressure are the same as those for pipeline crossover:
1) Open the emergency oxygen cylinder fully (not just the three or four quick turns used for checking).
2) Disconnect the pipeline connection at the wall because something is wrong with the oxygen pipeline.
3) Ventilate by hand with the anesthesia breathing circuit, rather than with the mechanical ventilator (which may use cylinder oxygen for the driving gas if the pipeline is unavailable).
How is RBC 2,3 DPG affected in blood transfusions?
Decreased 2, 3-DPG concentration shifts the dissociation curve to the left and decreases oxygen unloading to the tissues.
2, 3-DPG may decrease significantly during hypoxemia. It also decreases in the blood cell products during storage.
Red cell 2, 3-DPG in stored blood diminishes in storage and is effectively zero after 1-2 weeks (rate of decline depends on types of preservative used). After transfusion, red cell 2, 3-DPG returns to 50% after 7 hours and to normal level after 48 hours.
What is the best way to tell a patient to adjust their insulin (short, intermediate and long acting) in preparation for the morning of surgery?
Dosage Adjustments of Insulin Peri-operatively
Pre-operatively, patients on rapid or short-acting insulin can continue it till the day of surgery without any other changes.
Those taking intermediate* insulin (usually twice a day dose) *should take only 75% of the normal dose the night before surgery* and *50% of the normal dose the day of the surgery.
With long-acting insulin, the patient needs to reduce their dose by 50% on the morning of the surgery.
What is the current recommendation for Metformin in the perioperative setting?
This has led to the recommendation that metformin be continued until the day of the surgery.
The exception is patients with renal dysfunction, who will need to discontinue the metformin 24-48 hours prior to surgery.
- Compare solubility of the volatile anesthetic to uptake into the bloodstream.
- Then, compare the uptake to Rate of Rise of FA/Fi ratio?
The greater the solubility (Ex: Isoflurane is most soluble) of an inhaled anesthetic, the more rapid the uptake into the bloodstream
- The greater the uptake, the slower the rate of rise of FA/FI
(Ex: Hence why Iso is so slow)
Uptake of volatile anesthetics is directly proportional to what three factors?
- Cardiac output (Low Cardiac output will increase rate of rise but only with Iso)
- Solubility (Iso vs. Des)
- Alveolar-to-venous partial pressure difference
(#3 Ex: Low (PArterial - PVenous), meaning less blood uptake
A high pulmonary artery to pulmonary vein gradient means high solubility in the blood. A higher blood solubility means lower diffusion into the CNS, which slows onset.)
What is the most common blood reaction?
What is the definition of this subtype of blood reaction?
Explain the pathophysiology of this reaction.
Non-hemolytic febrile transfusion reaction
Non-hemolytic reaction is defined as a temperature increase of >1 °C without concurrent hemolysis.
A non-hemolytic febrile transfusion reaction occurs because recipient antibodies cause lysis of donor leukocytes found in the red cell transfusion product.
What is the pathophysiology of TRALI?
Antibodies in the donor blood bind to the recipient’s leukocytes, which then adhere to the vascular capillary bed in the pulmonary circulation.
This causes damage and extravasation of intravascular fluid.

What is the mechanism of Transfusion-Related Immunomodulation?
Transfusion of soluble leukocyte mediators
What is the mechanism of action of Tirofiban?
GP IIb-IIIa receptor inhibitors that prevent platelet aggregation and thrombus formation.
What is the mechanism of action of Ticlopidine?
adenosine diphosphate (ADP) receptor inhibitors which impair the ADP-dependent activation of the GP IIb-IIIa complex.
What is the mechanism of action of Eptifibatide?
GP IIb-IIIa receptor inhibitors and thus prevent platelet aggregation and thrombus formation.
Phase II of Action Potential.
What direction is K and Ca going?
K = Always efflux
Ca = Influx

What are 5 potential common causes of metabolic alkalosis?
Excessive bicarbonate administration
Conversion (such as citrate or lactate to HCO3 by the liver)
Excessive chloride loss such as with nasogastric suctioning
Increased renal reabsorption (hyperaldosteronism)
Volume contraction (as seen with diuretic therapy).
What are the potential complications with having a metabolic alkalosis?
Ex: CV, Pulm, Electrolytes
CV: Cardiac depression, Arrhythmia and vasoconstriction
Pulm: Impaired central respiratory drive and Difficult ventilator weaning
Hypokalemia
What is the treatment for metabolic alkalosis?
Treatment involves correction of the cause
(e.g. volume resuscitation with normal saline increases chloride, which increases renal bicarbonate losses)
Acetazolamide (promotes renal bicarbonate loss)
Infusions of acid such as ammonium chloride
Dialysis.
What is the general rule of thumb when considering compensation for metabolic alkalosis (i.e. What should PaCO2 be)?
What is the threshold for PaCO2 (Ceiling)?
A general rule of thumb is that for every 1 mEq/L increase in serum bicarbonate, the PaCO2 should increase by 0.5 mm Hg.
This mechanism is limited by stimulation of peripheral chemoreceptors to hypoxemia and thus PaCO2 rarely increases above 55 mm Hg.
What is the Bainbridge Reflex?
What is the reverse brainbridge reflex, and when is this seen clinically?
The Bainbridge reflex increases heart rate* by inhibiting parasympathetic activity when stretch receptors located in the *right atrial wall sense increased pressure.
Reverse Bainbridge Reflex during after spinal
More common in patients with high baseline vagal tone, anesthetic levels above T5, and is associated with decreased cardiac preload
What is the Hering-Breuer reflex?
Prevents overinflation of the lungs
When subjected to excessive stretching (e.g. during CPAP or PEEP), pulmonary stretch receptors trigger the reflex which temporarily prevents inspiration and allows expiration to occur.

Autonomic hyperreflexia:
When does it begin (Timeframe)?
When does present itself in the OR?
85% of cases occur at what spinal cord injury level?
Begins 2-3 weeks following acute injury.
It may occur with stimulation below the level of spinal cord injury leading to uninhibited sympathetic stimulation
Approximately 85% of cases occur with SCI above the T5 level.

What is the usual dosing of nitric oxide for RV dysfunction after cardiac surgery?
Usual dose: 20 ppm via mechanical ventilation circuit; dosage range: 5 to 20 ppm
What is unique when comparing central sleep apnea to obstructive sleep apnea?
Unlike in OSA, there is no effort to breathe during periods of apnea in CSA.
Thus one of the classic signs of OSA, snoring, does not commonly occur in patients with pure CSA.
Most cases of CSA are secondary to an underlying medical condition (e.g., heart failure, stroke, certain neuromuscular disorders), narcotic use, or altitude changes.
How is OSA defined?
≥5 episodes per hour of sleep of complete cessation of airflow during breathing lasting ≥10 seconds despite maintenance of neuromuscular ventilatory effort, accompanied by an SaO2 decrease of at ≥4%
How do we stratify the severity of OSA?
The total number of episodes of apnea or hypopnea* (reduction of airflow of greater than 50%) divided by the *total sleep time creates a apnea/hypopnea index (AHI).
An AHI of 5-15 events per hour indicates mild disease
An AHI of 15-30 indicates moderate disease
Severe disease is indicated by an AHI of >30.
What are the 3 phases in coagulation?
1) Primary hemostasis: platelets form a clot to plug vascular injury.
2) Coagulation: fibrin forms mesh over the clot to stabilize it.
3) Fibrinolysis: after the injury is repaired, the clot is broken down.

What phases of coagulation are affected by Liver Disease?
Does liver disease create a procoagulant or anticoagulant disease process?
Liver disease affects all 3 phases (Hemostasis, Coagulation, Fibrinolysis).
Patients with advanced liver disease have dysfunction of BOTH procoagulants and anticoagulants. This creates a balance in the coagulation system and these patients rarely have spontaneous bleeding or clotting.
What are the 4 ways you can measure the primary hemostasis phase?
1. Platelet count
2. Platelet function analysis
3. Von Willebrand Factor (vWF)
4. Bleeding time
What is the appropriate platelet count in Liver Disease, and why?
Appropriate platelet count is necessary to generate adequate thrombin levels. This threshold is thought to be around 50-60.
What are the two coagulation factors that are unaffected by Liver disease?
Factor VIII and vWF, are produced in the endothelial cells and are not reduced in liver disease. In fact, they are increased.
When does Liver disease become procoagulopathic?
They are NOT at increased risk for spontaneous bleeding. This is because in liver disease the procoagulants and anticoagulants are both decreased. As long as there is adequate platelet count, patients with liver disease will continue to generate thrombin and fibrin. However, as the liver disease advances, it becomes more difficult to maintain hemostasis even with the balance that has been established. Some shift towards clotting, and others towards bleeding.
What are the goals for coagulation in patients with liver disease:
PLT count?
Fibrinogen?
Hgb?
When to administer FFP?
Coagulation management in liver disease patients:
1) Maintain platelet count at 50-60; in high-risk surgery maintain >100
2) Keep fibrinogen >100
3) Transfuse to maintain Hgb > 7
4) Do not give FFP prophylactically or chase INR levels
- Increased INR in these patients does not necessarily reflect risk of bleeding
- If FFP is to be given, dose is 20-40 mL/kg
What is the preferred lead combination for detecting ischemia:
If given one lead?
If given 2 leads?
One lead = V5 (75%)
Two Leads = II and V4 (82%)
Intraoperative monitoring of Leads II and V4 is the preferred lead combination as it allows for rhythm monitoring and is sensitive for myocardial ischemia. Alone, V5 has the highest sensitivity for myocardial ischemia, however, this is not recommended for intraoperative monitoring.

How does Meperidine exhibit analgesic effects?
Meperidine’s beneficial effects are multimodal and center around the kappa opioid receptor.
When performing a central line with manometry, what is the expected height it should go up to?
See bold for answer
Pressure measurement via simple manometry, also referred to as tube or column manometry, is performed by attaching tubing to the thin-walled needle or the catheter that has been inserted into the presumed central venous site prior to dilation.
This tubing is then held below the level of the vein, allowing blood to fill the tubing followed by raising it vertically above the patient against gravity.
The blood-air interface will settle at the level where hydrostatic pressure in the tubing is equal to the intravascular pressure. If it is misplaced in the artery, the blood will rise out the top of the tubing.Recall that a pressure of 10 mmHg correlates with a height of 15cm.
Mag levels (Obstetrics)
What are the 2 indications?
What is normal?
Indications:
- Seizure Prophylaxis for preeclampsia
- Tocolytic agent in preterm labor
- relaxing uterine smooth muscle and vascular tone
1.8 - 2.5 mg/dL Normal
What level of magnesium is therapeutic for OB floor?
What symptoms can be seen with this?
4.8 - 9.6 mg/dL Therapeutic for obstetric floor
Flushing, nausea, vomiting, sedation, dizziness, and muscle weakness
Muscle weakness results from both a decreased release of acetylcholine (secondary to presynaptic calcium channel blockade) as well as decreased motor end plate sensitivity to acetylcholine.
A patient with therapeutic mag level needs crash general for caesarian section. What do you need to keep in mind for paralytic dosing for depolarizing* vs. *non-depolarizing?
Patient has hypermagnesemia therefore hypocalcemia
If these patients need general anesthesia, one must be aware that they may not have fasciculations with succinylcholine, and they will have an increased sensitivity to nondepolarizing neuromuscular blockers necessitating a decreased dose
When do ECG changes occur in hypermagnesemia?
What are the ECG symptoms to look for?
6 - 12 mg/dL ECG changes begin
Prolonged PR interval, widened QRS, Look for heart blocks
Hypermagesemia:
Loss of DTR and respiratory depression?
SA, AV, or Complete HB?
Code?
12 mg/dL Loss of Deep Tendon Reflexes, Respiratory Depression
18 mg/dL The SA and AV node blocked → complete heart block
20-25 mg/dL Cardiac arrest (asystole)
The ASA Taskforce released 2006 guidelines (5 total) for when FFP should be administered. Whata are they?
- Correction of excessive microvascular bleeding in the presence of a INR > 2
-
2._ Correction of excessive _microvascular bleeding secondary to coagulation factor deficiency* in patients transfused with more than one blood volume
3. Urgent reversal of warfarin therapy
4. Correction of known coagulation factor deficiencies for which specific concentrates are not available
5. For heparin resistance
How much FFP should be given to reduce warfarin? (Dose)
5 - 8 mL/kg then recheck values to guide resuscitation
What routes should be used for Vitamin K and why?
How long does this take effect?
PO = Most pre-dictable
IV = Can have anaphylaxis
SQ/IM not recommended
24 hours to take effect regardless of route
What are the two different types of PCC?
It should be noted that some products available in the U.S. contain negligible amounts of factor VII which is key for clotting in trauma patients (these products are known as three-factor PCCs).
Four-factor PCC contains higher amounts of factor VII.
What do you do for:
Urgent Surgery (<24 hours) with INR <1.9?
Warfarin Reversal, Urgent Surgery:
INR 1.5 - 1.9: consider PCC, FFP may not be useful
What do you do for:
Urgent Surgery (<24 hours) with INR 3?
INR 1.9 - 5:
PCC or FFP
1-3 mg of IV vitamin K
Kcentra, Beriplex P/N [Canadian product]: Vitamin K antagonist (VKA) reversal in patients with acute major bleeding or need for an urgent surgery/invasive procedure:
IV: Individualize dosing based on current pre-dose INR. Dosage is expressed in units of factor IX activity. Administer with vitamin K concurrently. Repeat dosing is not recommended (has not been studied).
Pretreatment INR: 2 to <4: Administer 25 units/kg; maximum dose: 2,500 units
Pretreatment INR: 4 to 6: Administer 35 units/kg; maximum dose: 3,500 units
Pretreatment INR: >6: Administer 50 units/kg; maximum dose: 5,000 units
What do you do for:
Urgent Surgery (<24 hours) with INR 7?
- *PCC**: 50 U/kg (Max dose of 5000 units)
- *FFP** (5-8 cc/kg)
Vitamin K 2-5 mg IV over 30 minutes
How do you administer PCC through an IV?
Kcentra, Beriplex P/N [Canadian product]:
Administer at room temperature at a rate of 0.12 mL/kg/minute (~3 units/kg/minute); do not exceed 8.4 mL/minute (~210 units/minute).
Do not allow blood to enter into syringe (fibrin clot formation may occur).
What do you do for surgery in 24-48 hours for:
INR of 1.7?
For INR 1.5 - 1.9
1 mg PO Vitamin K
What do you do for surgery in 24-48 hours for:
INR of 2.3?
What do you do for surgery in 24-48 hours for:
INR of 2.6?
INR 1.9 - 5:
1 - 2.5 mg PO vitamin K*, if INR still elevated 24 hours after dose give *1 - 2 mg PO vitamin K
What do you for non-emergent craniotomy for INR or 6.4
INR 5 - 9
2.5 - 5 mg PO vitamin K, if INR still elevated 24 hours after dose give 1 - 2 mg PO vitamin K
If you are on APS team and have a neuraxial catheter left in place, how often can you administer prophylactic lovenox?
Do not administer twice daily prophylactic doses of LMWH while a neuraxial catheter is in place.
You may administer once daily prophylactic doses of LMWH while a neuraxial catheter is in place starting 12 hours after needle insertion.
A patient has been receiving LMWH, how long after the last dose of LMWH can I perform a neuraxial procedure?
For patients that were receiving therapeutic doses (1 mg/kg) of LMWH, wait 24 hours after the last dose.
For patients that were receiving prophylactic doses (30-40 mg BID) of LMWH, wait 12 hours after the last dose.
I performed a neuraxial procedure where I left a catheter, can I start/restart LMWH while the catheter is in place?
Do not administer therapeutic doses of LMWH while a neuraxial catheter is in place.
Do not administer twice daily prophylactic doses of LMWH while a neuraxial catheter is in place.
You may administer once daily prophylactic doses of LMWH while a neuraxial catheter is in place starting 12 hours after needle insertion.
A patient with a neuraxial catheter in place has been receiving once-daily prophylactic doses of LMWH, when can I remove the catheter?
12 hours after last LMWH dose
A patient had a neuraxial catheter which was removed, how soon can I start/restart LMWH after catheter removal?
4 hours
Differentiate the types of characteristics of Phase 1 vs. Phase 2 Blocks:
- Typical Dosing / Infusion Duration
- Rocuronium administration effects on the block
- Train of 4 Ratio
- Response to tetanic stimulation
- Post-tetanic Facilitation

Epidural Spread affected by what factors? (6)
Age
Site of Injection
Position
Airway Pressure
Local Anesthetic Concentration
Local Anesthetic Volume
Why and how is age a determinant of epidural spread?
- Older = Greater spread
Decreased compliance of epidural space
Therefore space will expand less when LA injected thus cranial & caudal spread increases
Less fat in epidural space in older patients so spread is more
How does site of injection affect epidural spread?
Thoracic spread caudally
Low Thoracic spread cephalad
Mid thoracic spread both directions
How does positioning a patient for epidural affect spread?
Lateral = More spread (Vs. Sitting)
Trendelenburg results in greater spread of local anesthetics
How does an intubated patient affect epidural spread?
Airway Pressure
Mechanical ventilation with CPAP increases spread
Secondary to compression of epidural space from increased intrathoracic pressure
How does concentration and volume affect epidural spread?
Local Anesthetic Concentration
Increase block height with higher doses
Also affects block density/intensity
Local Anesthetic Volume
Most significant effect on epidural spread and the number of segments blocked
Nicardipine
Metabolism and Elimination?
Metabolism: Liver
Elimination: GI Tract - Bile and Feces
How do you determine how much is left in an oxygen E cylinder?
E cylinder Roughly 2000 psi for 660 L
Direct Relationship
I.E. 500 psi = (25% of 660L) = 165 Liters
What are the treatments for acute dystonic reactions?
1. Anticholinergic medications
Help restore balance to the dopaminergic and cholinergic balance
Preferred
Diphenhydramine (Both anticholinergic and antihistamine)
Benadryl 12.5 - 50 mg IV
Benztropine
Initial dose: IM, IV, Oral: 1 to 2 mg once.
Subsequent doses: Oral: 1 to 2 mg 1 to 2 times daily.
3-8 all per True Learn but couldn’t find doses
Anticholinergic medications such as diphenhydramine (recall that it is both anticholinergic and antihistamine) and benztropine are considered first-line therapies for EPS since they can rapidly (five minutes or less when administered IV) improve and/or eliminate symptoms. They work by restoring the dopaminergic-cholinergic balance.
- Trihexyphenidyl
- Atropine
- Anti-histamines
- Benzodiazepines (Refractory to anticholinergics or when anticholinergics are contraindicated)
Directly relieve muscle spasms
- Beta-Blockers (Refractory to anticholinergics or when anticholinergics are contraindicated)
- Dopamine Receptor Agonist
- Alpha Adrenergic Agonists
What are the risk factors for latex allergies?
Multiple Surgeries
Healthcare workers
Atopic Histories
Tropic Fruits - Avocado, Kiwi, Banana, Chestnuts, Stone Fruits
What are the 4 grades of anaphylaxis?
Grade I involves cutaneous-mucous signs
Grade II corresponds to mild cutaneous-mucous features that may be associated with cardiovascular and/or respiratory signs
Grade I and II non-life threatening
Cardinal sign of grade III is cardiovascular collapse that may be associated with cutaneous-mucous signs and/or bronchospasm
Grade IV is cardiac arrest
When should you draw a tryptase level?
Elevated in Anaphylaxis
Peak between 15-60 minutes in Grade I and II
Peak between 30 - 120 minutes in Grade III/IV reactions
Half Life of tryptase = 2 hours
What do you need to keep in mind with SSRI medications when taking care of a chronic pain patient?
SSRIs inhibit CYP2D6 which reduces the activation of many opioids (e.g. codeine, hydrocodone oxycodone) to more potent forms (e.g. morphine, hydromorphone, oxymorphone).
What are the metabolites of Codeine, Hydrocodone and Oxycodone, respectively?
Codeine → Morphine
Hydrocodone → Hydromorphone
Oxycodone → Oxymorphone
Which is stronger, hydrocodone or oxycodone?
Hydrocodone → Hydromorphone
Slightly weaker analgesic than morphine
Conversion increases analgesic potency by a factor of 4
Oxycodone → Oxymorphone
Slightly stronger than morphine
Conversion increases its analgesic potency by a factor of 2
What is the effect of vasopressin on platelets?
Decrease number and causes aggregation
You have a case where upper extremity tourniquet will be applied and you are planning on a supraclavicular block.
- What will be blocked?
- What will not be blocked?
- What should you do to block what won’t be blocked?
Blocks the musculocutaneous, radial, ulnar, and median nerves
Fails to block the medial aspect of the upper arm supplied by the intercostobrachial nerve* (not a branch of the brachial plexus) and therefore supplementation of the supraclavicular block with an *intercostobrachial nerve block may be needed to prevent tourniquet pain.

Vascular surgery needs to create an AV fistula in the forearm. What regional technique could you consider?
Block the lateral & Medial antebrachial cutaneous nerve

What is the order of of fluoride ion production of volatile anesthetics?
(Rank them)
Desflurane
Enflurane
Isoflurane
Methoxyflurane
Sevofluranae
Methoxyflurane created the largest concentrations of inorganic fluoride ions and fluoride-induced nephrotoxicity. Enflurane was related to renal concentration effects of fluoride ions causing nephrotoxicity. Sevoflurane produces more fluoride ions than enflurane while being administered, but the concentration of fluoride rapidly declines with cessation of administration, owing to its low blood and tissue solubility.
Fluoride ion production: methoxyflurane > sevoflurane > enflurane > isoflurane > desflurane.
What is the dose and route of terbutaline?
Dosing: SubQ: 0.25 mg/dose;
may repeat every 20 minutes for 3 doses (maximum: 0.75 mg/1-hour period) (NAEPP 2007)
When dealing with post partum hemorrhage, what is the incremental dosing for oxytocin?
3U load and begin infusion at 18U/hour
Repeat 3U loads every 3 minutes and increase gtt to 46U/hour
When dealing with post partum hemorrhage, what is the incremental dosing for methergine?
0.2 mg IM
q20 min x2 then q2-4 hours
When dealing with post partum hemorrhage, what is the incremental dosing for carboprost?
0.25 mg IM
q20 min don’t exceed 8 doses in 24 hours
What are the side effects of methergine?
HTN
Headache
N/V
Pulmonary Hypertension
Chest Tightness
What are the side effects of carboprost?
N/V
Flushing
Bronchospasm
Diarrhea
Pulmonary Hypertension
When dealing with post partum hemorrhage, what is the incremental dosing for cytotec?
Onset?
Side effects?
600 - 1000 mcg buccal mucosa/OR
10 minute onset
SE: 38.1 Temp anad N/V, diarrheaa
Anaphylactoid Reactions:
How do they differ clinically from anaphylaxis?
Mechanism:
Triggers?
Tryptase levels?
Clinically indistinguishable from anaphylaxis
Mechanism: Uncontrolled histamine release by a mechanism not involving IgE.
Triggers: Protamine, IV contrast dye, opioids, d-tubocurarine, and thiopental.
Tryptase will rise
How does volatile anesthetics affect neuromuscular blockade?
Volatile anesthetics potentiate (Increase) neuromuscular blockade by DECREASING sensitivity of the postjunctional membrane to depolarization* and *INCREASING skeletal muscle blood flow which both augment neuromuscular blockade.
In addition, potentiation of neuromuscular blockade occurs by depression of upper motor neurons.
What is the only established clinical effect of TRIM?
Renal Allografts enhanced survival - The only established clinical effect of TRIM
What is the alveolar gas equation?
PAO2 = [(Patm – PH2O) * FiO2] – (PACO2 / R)
Where:
PAO2 = alveolar oxygen tension
Patm = barometric pressure
PH2O = water vapor pressure
FiO2 = percent of inspired oxygen
PACO2 = alveolar carbon dioxide tension
R = respiratory quotient (CO2 produced per O2 consumed).
What is a normal A-a gradient?
What is a Normal A:a ratio?
A normal A-a gradient is < 10 mm Hg
normal a-A ratio is >0.75.
You are doing a carotid endarterectomy and the patient suddenly goes into cardiac arrest. What was the likely reason?
Pressure on Carotid Sinus
Sensory fibers increased that send action potentials to the cardioregulatory and vasomotor centers in the medulla oblongata
(See image below)

How do you dose Lorazepam?
In doses of 25-50 mcg/kg (max dose of 4 mg) significant amnesia may remain up to 4-6 hours after a dose without excessive sedation, but above 4 mg there is no added benefit and it will prolong sedation.
IV: 1 to 4 mg or 20-40 mcg/kg (maximum single dose: 4 mg) once 5 to 20 minutes before procedure; if needed based on incomplete response and/or duration of procedure, may repeat the dose (usually at 50% of the initial dose) after ≥5 minutes
What is the difference in duration of midazolam vs. lorazepam?
Midazolam = 2 hours
Lorazepam = 6-8 hours
What is the first step in management during loss of pipeline pressure?
Management of pipeline failure or gas crossover:
1) Disconnect the pipeline connection at the wall.
2) Open the emergency oxygen cylinder fully.
3) Ventilate by hand with the anesthesia breathing circuit.
How does calcium and magnesium levels affect depolarization doses of neuromuscular blockade?
Hypercalcemia –> Antagonizes non-depolarizing neuromuscular blockade therefore you need higher doses
Magnesium = Opposite
What are the benefits of Fospropofol?
Less respiratory depression (<10%) compared to prop (10-25%)
Onset is slower (4-10 minutes)
What are the two most common side effects of fospropofol?
1. Paresthesias >50% of patients (an abnormal sensation, typically tingling or pricking)
5 minutes after pushing
burning, tingling, and/or stinging sensations and usually occur in the perianal or genital regions
Can be buttock, chest, ears, nose
Resolve spontaneously
LA, NSAIDs, or opiates don’t decrease incidence
2. Pruritits 15-30% of patients (Genitals)
How does ventilation affect calcium?
Acidiotic –> more H+ attach to albumin –> Hypercalcemia
Alkalosis –> Less H+ attach to albumin –> Hypocalcemia

What are the signs of citrate toxicity?
Calcium levels?
BP?
Heart Rhythm?
Pulse Prressure?
LVEDP?
CVP?
The signs of citrate toxicity include those associated with hypocalcemia
Hypotension
Arrhythmias
narrow pulse pressure
Increased intraventricular end diastolic pressure
Increased central venous pressure
Look at V1.
What are the 6 diagnosis that this could be?

The following diagnoses should be considered in patients with a large R wave in lead V1:
1) Right ventricular hypertrophy (RVH)
2) Posterior wall MI
3) Wolff-Parkinson-White syndrome (WPW)
4) Muscular dystrophy
5) Right atrial enlargement (RAE)
6) Right ventricular strain with ST-T wave abnormalities
How does MAC change depending on sodium levels?
Hypernatremia = Increase MAC
Hyponatremia = Decrease MAC
How does MAC change with ETCO2?
Hypocarbia = Increase MAC
Hypercarbia = Decrease MAC
Describe the 2 routes that coronary blood flow can empty into (Think venous coronary circulation).
Coronary venous drainage ultimately converges in the coronary sinus, which subsequently empties into the posterior right atrium.
While 85% of coronary blood flow to the left ventricle empties into the coronary sinus
15% will eventually drain via Thebesian veins into the atrial and ventricular cavities.
What are the 3 main cardiac veins and what do run along side?
Anterior = RCA
Great = LAD
Middle = PDA

What nerves are responsible for sensation:
- Epiglottis to the vocal cords?
- Inferior to vocal cords and trachea?
Internal Branch of Superior Laryngeal Nerve (CN X) = Sensory above the cords
Recurrent Laryngeal Nerve (CN X) = Sensory below cords and Trachea
Of the Volatiles and Nitrous Oxide, rank the order of the gases in terms of aumentation of neuromuscular blockade.
Greatest to Least:
Desflurane (60% increase)
Isoflurane and Sevoflurane (40%)
Nitrous Oxide (20%)
Why is Thomboxane inhibition important with Aspirin?
Thromboxane
- Responsible for platelet aggregation (Aspirin blocks this)
The prevention of its formation by aspirin prevents platelet aggregation for the life of the platelet.
- Thromboxane is also a potent vasoconstrictor, and its inhibition may promote coronary perfusion.
What are the characteristics of an addicted anesthesiologist?
- *Characteristics of addicted anesthesiologists include:**
- 50 percent < 35 years old (bimodal distribution) Young
- Residents are over-represented. “Often they are the last individuals in your department you would expect.”
A high proportion are members of Alpha Omega Alpha.
- 67-88 percent are male
- 75-96 percent are White
- 76-90 percent opioids were the drug of choice
- 33-50 percent of polydrug users
- 33 percent with a family history of an addictive disease
- 65 percent were associated with academic departments (greater vigilance and younger age)
How does Lithium affect dosing of paralytics?
Lithium prolongs action of both depolarizing and nondepolarizing neuromuscular blockers.
What is is the only nondepolarizing neuromuscular blocking agent that is metabolized by pseudocholinesterase?
Mivacurium
How does higher altitute affect the outflow from vaporizer (output)?
Decreasing barometric pressure (higher output) will increase the output of the vaporizer.
What 4 lab tests can differentiate Pre-renal, intrinsic, vs. Post renal AKI?
- Urine Osmolality
- Urine Sodium
- FENa or FEUrea (if on diuretics)
- BUN/Creatinine
Using urine osmolality, urine electrolytes, BMP, and FENa, how would you diagnose between Prerenal, Intrinsic, and Post renal etiologies?
Prerenal: UOsm of >500, UNa of <10, FENa of <1%, BUN/Cr of >20
Intrinsic: UOsm of <350, UNa of >20, FENa >2%, BUN/Cr of <15
Post renal: UOsm of <350, UNa of >40, FENa of >4%, BUN/Cr of >15

How do you determine differences in ATN vs. Pre-renal syndrome>
The gold standard for the distinction between the two is the kidneys response to a fluid challenge.
If enough fluid is given to reverse signs of volume depletion and the serum creatinine does not return to baseline within 72 hours, ATN is the likely diagnosis.
What is pathognomonic for ATN?
The presence of “muddy brown casts” of epithelial cells found in the urine during urinalysis is pathognomonic for ATN
Tolvaptan:
Class/Mechanism?
Indication?
Mechanism: Vasopressin receptor type 2 (V2) antagonist.
Indications:
Tolvaptan was originally approved for use in patients with acute heart failure
Now used in patients with excessive antidiuretic hormone secretion (SIADH).
What is the average FRC volume in adults?
The average FRC in the adult is approximately 30 ml/kg
(~2100 mL for a 70 kg adult).
How does FRC change with semi-fowler positioning (60 degrees) to supine?
How does FRC change with trendelenburg?
The greatest decrease in functional residual capacity (FRC) occurs when going from 60° upright to supine (0°) or when being placed in Trendelenburg (head-down) more than -30°.
The FRC does not significantly decrease when changing from a supine position to Trendelenburg less than -30°.
However, beyond -30°, the drop in FRC is considerable
The FRC reduction when going from upright to supine or prone is approximately what percentage in a healthy adult?
10%
If you have a patient with Myasthenia Gravis, how do you adjust your doses of paralytic?
Less susceptible to SCh (Give a higher dose)
more susceptible to non-depolarizing NMBDs (Give a lower dose)
What are the 4 sites correlating with core temperature?
1) Esophageal
2) Tympanic
3) Pulmonary artery
4) Nasopharyngeal
What are the consequences of hypothermia in the intraoperative setting?
Consequences of Hypothermia
Coagulopathy
Increased infections
Changes in oxygen delivery
Increase in myocardial oxygen demand
Increase in blood transfusion requirements
Poor wound healing
Changes in drug metabolism
How would a storage of PRBC affect Hgb Dissociation Curve?
Hint: pH, 2,3-DPG, Potassium
Decrease in pH (Right shift)
Decrease in 2,3 DPG (Left shift)
Increase in Potassium
Potassium gradually moves out of PRBCs during storage in order to maintain electrochemical neutrality with the hydrogen ions that are generated from anaerobic metabolism. After three weeks of storage, potassium levels may reach 20-35 mEq/L*, though there is usually only 20-60 mL of plasma in one unit of PRBCs. After 4-5 weeks of storage, transfusion of one unit of PRBCs can add *5-7 mEq of potassium. Massive transfusions, particularly in neonates, can lead to hyperkalemia.

What is the time frame for which burn patients have contraindications to Succinylcholine (Before and after burn)?
After the first 24 hours following the burn injury and has implications for both depolarizing and nondepolarizing neuromuscular blocking drug use. When the burned area exceeds 10% of the total body surface area (TBSA), succinylcholine administration between 24 hours and 1 year after the injury becomes contraindicated due to exaggerated hyperkalemia.
For Burn patients, when do non-depolarizing neuromuscular blockers start develop resistance? When is the peak?
How does this change affect:
1. Onset?
2. Duration?
3. Effect?
Starting 1 week following the burn and peaking approximately 5-6 weeks following the injury.
The resistance is primarily due to the significant increase in the number of acetylcholine receptors but is also due to production of altered isoforms of the acetylcholine receptor, increased renal excretion, and altered serum protein binding of the drugs.
Resistance leads to an increased time to onset (slowed up to 30%), decreased duration of action, and decreased overall effect. These effects can be at least partially overcome by increasing the dosage of the drug
What is the therapy that goes from E to B?

Vasodilators
What is the therapy that goes from E to D?

Ionotropes and Vasodilators
What is happening from A to B?

Increase in preload (IVF bolus)
What is seen here?

What should you do?
Incompetent Expiratory Valve
1. Replace Exhausted CO2 absorbent
2. Increase gas flows
What is seen here?

Incompetent Inspiratory Valve
Butorphanol
- Mechanism?
- Indication?
Butorphanol
Mechanism:
Partial Opioid Agonist and Mixed Agonist-Antagonist
Synthetic mu agonist-antagonist opioid analgesic
Partial agonist activity at the κ-opioid receptor
Indications:
Relieve Biliary Colic
Side Effect: Does not cause biliary spasm, does not sufficiently contract sphincter of Oddi and increase common bile
What is the recommended precurarization dosage for a nondepolarizing agent?
The recommended precurarization dosage for a nondepolarizing agent is 10% of the ED95, given about 3-5 minutes prior to succinylcholine. Using 10% of the intubating dose (20% of the ED95) is associated with an unacceptably high rate of side effects, including dyspnea in an awake patient.
Rocuronium
ED 95 - 0.3 mg/kg
Precurarization - 0.03 mg/kg
How do SSEP demonstrate cerebral/cord ischemia?
1. Decreases in SSEP amplitude
2. Increases in latency are indicative of insults to the somatosensory pathway such as cerebral ischemia.

TEG shows decreased MA. What is the treatment?
Thromboelastography (TEG) is both a quantitative and graphic evaluation of clotting function. Decreased MA values primarily suggest quantitative and/or qualitative platelet dysfunction or, to a lesser extent, inadequate fibrinogen.

The best treatment is administration of platelets.
How does midazolam affect ventilation?
Midazolam and all benzodiazepines cause a dose dependent decrease in minute ventilation.
The decreased minute ventilation is mainly a result of decreased tidal volume.
Benzodiazepines decrease the respiratory sensitivity to CO2 and act synergistically with opioids.
How do benzodiazepines affect ventilation?
Decrease in tidal volume, which causes a decrease in minute ventilation.
The respiratory rate is often preserved, but with higher doses of medication apnea may occur.
The depression in minute ventilation seen after midazolam occurs rapidly (less than 5 minutes) and lasts for about 1 hour.
Synergistic with opioids to depress minute ventilation.
Which benzodiazepine has the highest affinity for GABA receptor?
Lorazepam
What are the following time windows when determining max dose local anesthetics?
Chloroprocaine
Lidocaine
Ropivicaine
Bupivicaine
Note, these maximum allowable doses are based on the duration of action and half-life of each local anesthetic (LA). The maximum allowable doses are for the following time windows:
Chloroprocaine: 30-60 minutes for 12 mg/kg
Lidocaine: 60-90 minutes for:
Lidocaine without Epi - 5 mg/kg
Lidocaine with Epi - 7 mg/kg
- *Ropivacaine**: 90-120 minutes for 3 mg/kg
- *Bupivacaine**: >120 minutes at 2.5 mg/kg
What are the risk factors for myalgia induced from succinylcholine?
More likely in females of child bearing age having minor surgery
What are the pre-treatments used to mitigate myalgia induced from succinylcholine?
- Pretreatment with a nondepolarizing muscle relaxant
2. Administration of lidocaine
3. NSAIDs have all been tested and found to decrease the incidence of myalgias from succinylcholine.
What specific nerve palsies arre most common with LMA?
What are the risk factors?
Lingual nerve, recurrent laryngeal nerve, and hypoglossal nerve.
Risk factors include:
Overinflation of a small-fitting cuff
Prolonged operative times (>2-4 hours)
Lidocaine lubrication
Difficult insertion
Use of nitrous oxide
Cervical joint disease
You have a CKD patient who is weak in the PACU.
What are methods you could do in order to make as rapid as possible the metabolism of cisatracurium?
Hofmann elimination reaction is a pH- and temperature-dependent reaction
Proceeds more readily when pH (alkalotic) and temperature are increased
Cisatracurium:
Intubating dose?
Duration of action?
Eliminiation Half-Life?
An intubating dose of cisatracurium (0.1-0.15 mg/kg)
Duration of action: 30-60 minutes
Elimination half-life: 22-25 minutes
What are the elative contraindications to the use of closed circuit or low flow anesthetic techniques include?
Relative contraindications to the use of closed circuit or low flow anesthetic techniques include:
sevoflurane use and patients with alcoholism, malnutrition, cirrhosis, or ketoacidosis.
What is the required FGF (oxygen) equation?
The required FGF (oxygen) can be determined by calculating the metabolic demand for oxygen, approximately 3-3.5 mL/kg/min (for adults) * patient weight in kilograms.
Ex: 100 kg guy should get 350 mL/min of oxygen flow
What is the minimum amount of time for dual anti-platelet therapy for Bare-Metal Stent vs. Drug Eluting Stents, respectively?
Stent type: Minimum duration of DAPT:
Bare-metal stent 30 days
Drug-eluting stent 3 month minimum, 6 month ideally
What are the independent risk factors for post operative cognitive dysfunction?
- Advancing age
- Lower educational level
- History of previous CVA w/o impairments
What is the succinylcholine dose used for laryngospasm?
Small doses of succinylcholine (e.g. 0.1-0.5 mg/kg) can be used.
Describe the anatomical landmarks of the Larson Manuver?
The Larson maneuver involves medial and cephalad pressure at the laryngospasm notch.
The notch is located just behind the earlobe, bordered by the base of the skull superiorly, the mastoid process posteriorly, and the ramus of the mandible anteriorly.

How is a diagnosis of carbon monoxide poisoning made?
The diagnosis of carbon monoxide poisoning is made by co-oximetry, when elevated levels of COHb (>15%) are found on arterial or venous blood gas analysis.
What is the dosing of lipid emulsion therapy for LAST?
A bolus of 1.5 mL/kg (20%) should be delivered over one minute followed by an infusion of 0.25 mL/kg/min.
The infusion should be run until at least 10 minutes following restoration of cardiovascular stability.
The infusion may be increased to 0.5 mL/kg/min in the setting of persistent hypotension
Bolus (1.5 mL/kg) may be repeated 2x in the setting of continued cardiovascular collapse
Why do we avoid vasopressin and use lower doses of epinephrine in LAST patients?
Epinephrine by itself is highly arrhythmogenic and these effects may be even more pronounced in the setting of LAST.
Animal studies do not support its use
Animal studies have shown that lipid-only resuscitation of bupivacaine toxicity had better outcomes than epinephrine-only resuscitation due to the presence of severe arrhythmias seen in the latter.
Despite this, the use of small doses of epinephrine is still recommended since epinephrine provides critical inotropic and vasopressor support.
What are the nerves responsible for laryngospasm?
(Include afferent and efferent)
Afferent Branch - Internal Branch of superior laryngeal nerve
The internal branch of the superior laryngeal nerve is responsible for sensory innervation of the trachea at and above the level of the vocal cords. This means that the internal branch of the superior laryngeal nerve functions as the afferent limb of the laryngospasm reflex.
Efferent Branch - Recurrent Laryngeal nerve
The efferent branch of the laryngospasm reflex is mediated by the recurrent laryngeal nerve. The recurrent laryngeal nerve innervates all the intrinsic muscles of the larynx, with the exception of the cricothyroid muscle. The lateral cricoarytenoid and transverse arytenoid muscles are major adductors of the vocal cords and responsible for laryngospasm

Explain the CVP in terms of the 3 waves and 2 descents.
The central venous pressure (CVP) tracing includes 3 waves (a, c, v) and 2 descents (x, y).
The a-wave is caused by right atrial contraction.
The c-wave occurs when the right ventricle contracts, causing the tricuspid valve to bulge into the right atrium.
The emptying of the right ventricle produces the x-descent.
The v-wave is due to the filling of the right atrium
y-descent is the passive emptying of the right atrium.

What are the two types of Acetylcholine receptors?
Where are each located, respectively?
Nicotinic
Skeletal muscle* and *autonomic ganglia
Muscarinic
End organ receptors on salivary/gastric glands
Smooth muscle [bronchial, gastrointestinal, bladder, and vascular]
SA and AV nodes of the heart
When you give an acetylcholinesterase inhibitor, what are you expecting to happen to end organs of:
Cardiac
Bradycardia and Hypotension
When you give an acetylcholinesterase inhibitor, what are you expecting to happen to end organs of:
Pulmonary
Bronchospasm
Increased respiratory secretions
When you give an acetylcholinesterase inhibitor, what are you expecting to happen to end organs of:
GI
Increased peristalsis, salivation, GI secretions
When you give an acetylcholinesterase inhibitor, what are you expecting to happen to end organs of:
Ophthalmic
Miosis
Decreased intraocular pressure
What are the reasons (Theorized) for potentiating neuromuscular blockade with excessive neostigmine?
- Excessive neostigmine may lead to a depolarizing block, similar to succinylcholine, because of excessive motor end plate depolarization by acetylcholine.
- In addition, some animal studies have shown that neostigmine might directly block the acetylcholine receptors in high doses.
- Lastly, neostigmine can potentiate the effect of succinylcholine by inhibiting pseudocholinesterase
Fentanyl Transdermal Patch
Onset?
Time to Peak Plasma Concentration?
Time takes to get to 50% after removal?
Onset = 6-8 hours
Peaks = 30 hours
24 hours to get to 50% (not easily titratable)
Mortality rate for physicians re-entering practice after being treated for substance abuse is what percent?
For those re-entering training, mortality is what percent?
Mortality rate for physicians re-entering practice after being treated for substance abuse is between 9-13%. For those re-entering training, mortality is 7%.
The central tendency of interval data sets, especially those that are not normally distributed, is most accurately represented by what?
Median
What type of data sets refer to categorically discrete data sets, such as the name of the anesthesia delivered to a patient (e.g. general, regional, local)?
Nominal data sets refer to categorically discrete data sets, such as the name of the anesthesia delivered to a patient (e.g. general, regional, local).
Remember, NOMINAL = NAME. The mode, which is a measure of the most frequent value in the data set, is the only measure of central tendency for nominal data sets.
What test is used when comparing interval data in three or more groups?
One-way analysis of variance (ANOVA)
What is the Poiseuille Law Equation?
The Poiseuille Law is determined by the equation
Q = (P*pi*r4)/(8*n*l)
where Q = flow rate, P = pressure, r = radius, n = viscosity and l = length of tubing
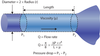
What impotant physics principle does NOT affect Poiseuille Law?
The density of the fluid is not part of the equation and does not directly affect laminar flow rate.
Density is not directly related to Poiseuille’s Law, however, it does help predict if fluid will flow in a laminar or turbulent manner. Poiseuille’s Law only relates to fluid flow that is laminar.

In newborns, where does the conus and dural sac end?
In adults?
In newborns, the dural sac typically ends at S3 and the conus medullaris at L3.
In adults, the dural sac typically ends at S1-S2 and the conus medullaris at L1-L2.
What Reynolds number will predict turbulent flow (vs. laminar)?
Typically, fluid with a Reynolds number greater than 4000 will be turbulent, less than 2300 is laminar, and between is a transition where either can occur depending on other factors such as the roughness of the tubing or plaque in blood vessels.
What test is used for comparison of two or more populations with respect to a single variable with categorical data (nominal scale)?
Chi-square testing
Garlic, ginger, gingko, and vitamin E have all been shown to interfere with what?
Platelet Function
What are the effects of glycopyrolate on:
Gastric Emptying
Salivary secretions
Gastric secretions
HR
Bronchial Smooth Muscle
LES tone
GU system
Glycopyrrolate delays gastric emptying, decreases salivary and gastric secretions, increases heart rate, relaxes bronchial smooth muscle, decreases lower esophageal sphincter tone, and causes urinary retention
How long does glycopyrolate last?
The duration of action of glycopyrrolate is 2-4 hours so if given shortly before anesthetic induction, the risk of aspiration is slightly increased due to the gastrointestinal side effects discussed above.
Which of the following lists the order of volatile anesthetic metabolism, from greatest to least?
Sevo > Iso > Des
Sevoflurane undergoes the most extensive metabolism of the inhaled anesthetics used in clinical practice today. Despite demonstrated elevations in serum fluoride concentrations in long cases, it has not been shown to cause fluoride-induced nephrotoxicity.
What is the bolus dose of hypocalcemia based on weight?
An IV bolus dose of 7 mg/kg may last as long as 10-15 minutes.
What is the equiation for left coronary perfusion pressure?
CPP = AoDP - LVEDP
CPP is coronary perfusion pressure, AoDP is aortic diastolic pressure, and LVEDP is left ventricular end diastolic pressure.
Which of the following breathing systems is the most efficient user of fresh gas flow during spontaneous ventilation?
Mapleson A is the most efficient user of FGF during spontaneous ventilation, but is the least efficient for controlled ventilation.
Here is a mnemonic to help remember the most useful circuits for spontaneous or controlled ventilation:
\
All Dogs Can Bite (spontaneously): A > D > C > B
Dead Bodies Can’t Argue (controlled): D > B > C > A
Spontaneous = A
Controlled = D
What type of Circuit is a Jackson Reese?
What should you put your flows (controlled) vs. (spontaneous)?
The Mapleson F system (also known as the Jackson-Rees modification) is relatively efficient at both spontaneous ventilation (FGF of 2-3 times MV*) and *controlled ventilation (FGF approximately 2 times MV)
What should your gas flows be for a Bain Mapleson D system?
(Controlled and Spontaneous)
The Mapleson D system with the Bain modification has the fresh gas inflow tubing running coaxially inside the corrugated breathing tubing. This modification makes it very efficient for CONTROLLED ventilation, typically requiring FGF only 1-2 times MV.
Spontaneous ventilation requires FGF of 1.5-3 times MV to prevent rebreathing.
How do you stratify patients are adrenally suppressed?
Low-risk patients are those who taking < 5 mg/day of prednisone (or equivalent) regardless of the time duration or patients on glucocorticoid therapy for < 3 weeks regardless of dose.
High-risk patients are those taking > 20 mg/day of prednisone (or equivalent) for > 3 weeks or those with cushingoid symptoms.
Intermediate-risk patients are those taking 5-20 mg/day of prednisone (or equivalent) for > 3 weeks; those who have discontinued glucocorticoid use within the past year; or those who use chronic inhaled or topical glucocorticoids.
What is the pathway for the oculocardiac reflex?
Stimuli at the eye -> ciliary ganglion -> ophthalmic division of trigeminal nerve -> Gasserian ganglion -> trigeminal nucleus -> vagus nerve -> bradycardia.

What is Leukoreduction?
What can this reduce the risk of?
What does i not fully protect against?
Leukoreduction is a decrease or complete elimination of donor leukocytes in blood components.
This can reduce the risk of febrile transfusion reactions and the risk of viral transmission (e.g. cytomegalovirus).
It does not fully protect against bacterial infection and does not prevent graft-versus-host disease.
What are the cardiopulmonary effects of isoflurane at 1.0 MAC?
(HR, BP, SVR, CVP)
(MV, TV, RR)
At 1.0 MAC of isoflurane, cardiovascular effects include increased heart rate (10-15 beats/min), decreased SVR and blood pressure, and minimal changes in CVP.
Respiratory effects include a mild reduction in minute ventilation, a reduced tidal volume, and an increased respiratory rate.
What patients and food allergies are increased allegy to latex?
Health care workers, atopic individuals, and those with allergies to certain foods (avocados, bananas, chestnuts, kiwi fruit, papayas, potatoes, tomatoes) have an increased incidence of latex allergy.
What is the mechanism of Nalbuphine?
What does this exhibit a plateau of?
Nalbuphine is a mu antagonist and kappa opioid receptor agonist which exhibits a plateau effect for respiratory depression.
Angiotensin II (A2)-mediated vasoconstriction is produced within how many minutes after the onset of acute hypotension or hypovolemia?
20 minutes
The release of A2 is modulated by the renin-angiotensin-aldosterone system (RAAS).
Blockade of the RAAS by angiotensin-converting enzyme inhibitors or angiotensin receptor blockers may cause profound and refractory perioperative hypotension, particularly after induction of general anesthesia.
What is a normal wedge pressure?
Normal pulmonary wedge pressure is 6-12 mmHg
Whats the size of an E cylinder oxygen tank?
Volume and psi?
A “full” E size oxygen cylinder is variously quoted in the anesthesia literature as being pressurized to 1900, 2000, or 2200 psig.
Similarly, major anesthesia textbooks differ in regards to how many liters of oxygen are in a “full” E size oxygen cylinder, most often stating it to be 600, 625, or 660.
What are the important factors for spinal spread?
Epidural spread?
Drug dosage, drug baricity, and patient positioning are the most important factors influencing level of spinal blockade.
Drug volume is an important factor during epidural blockade.
What percentage of anesthesiology residents who were found to have substance use disorder during training relapsed?
At least 40% of anesthesiology residents who were found to have substance use disorder during training relapsed.
Preservation of Total Hepatic Blood Flow amongst volatile anesthetics at 1 MAC, from greatest to least is?
Sevoflurane > isoflurane > halothane.
How much suppression of ACh receptors are blocked by paralytic with:
1
2
3
4
Palpated twitches
1 palpated twitch indicates >90% suppression.
2 palpated twitches indicate 80-90% suppression.
3 palpated twitches indicate 70-80% suppression.
4 palpated twitches indicate up to 65-75% suppression.
Palpation of twitches using TOF testing cannot detect the percentage of receptors bound at < 65%.
What drug can you give besides benzodiazepines that can decease post operative delirium with ketamine?
Physostigmine (0.5 - 2.5 mg IV) may be able to reverse the emergence reactions that are seen with ketamine.
Physostigmine is a centrally acting acetylcholinesterase inhibitor that will increase the amount of acetylcholine in the brain.
The theory behind this mechanism of reversal is that ketamine’s emergence reactions resemble the central anticholinergic syndrome thus, giving physostigmine may shorten the response. However, data is conflicting regarding the reliability of physostigmine for reversal of symptoms.
What are the Risk Factorsr for emergence reactions with Ketamine?
Factors that influence incidence of emergence reactions:
Age: Adult patients are more likely to report a high incidence compared to pediatrics.
Gender: Females are more likely than males.
Dosage: Larger doses with rapid administration increase the risk.
Psychological Susceptibility: Certain personalities types are at an increased risk; those who tend to dream at home and those who score high on the Eysenck Personality Questionnaire (extrovert, neurotic) tend to have a higher risk.
Concurrent Medications: Multiple medications increase the incidence although administering a benzodiazepine prior to administration of ketamine helps decrease the risk.
What is the equation of Compliance of the Respiratory system?
1/C(Resp System) = 1/C (Lungs) + 1/C (Chest wall)
Where C is compliance, RS is respiratory system, L is lungs, and CW is chest wall.
FiO2 of simple face mask?
What is the FiO2 of a nasal cannula?
A simple face mask supplies an FiO2 of approximately 35-50% with oxygen flows of 5-10 L/min.
Nasal cannulas provide an FiO2 of 25-40% with flow rates up to 6 L/min.
What is an IM dose of phenylephrine?
An IM dose of 2-5 mg will raise blood pressure and lower heart rate typically within 10-15 minutes from injection.
Ketorolac Mechanism of Action on the kidneys?
Ketorolac = Vasoconstriction of afferent glomerular arterioles.
Prostaglandins dilate afferent arterioles and increase glomerular capillary perfusion pressure. Ketorolac (and other NSAIDs) inhibit this vasodilatory effect and result in afferent arteriolar vasoconstriction.

The chemoreceptors are stimulated to increase minute ventilation in response to decreases in PaO2 below what value?
60-65 mmHg of PaO2
The ASA has a published algorithm for difficult airway, which lists 11 non-reassuring findings on physical exam that predict difficulty with intubation.
What are they?
1) Relatively long incisors
2) Prominent “overbite”
3) Patient cannot bring mandibular incisors anterior to maxillary incisors
4) Less than 3 cm interincisor distance
5) Uvula is not visible when tongue is protruded with patient in sitting position
6) Highly arched or very narrow palate
7) Mandibular space that is stiff, indurated or occupied by a mass
8) Less than three ordinary finger breadth thyromental distance
9) Short neck length
10) Thick neck circumference
11) Decreased extension or flexion of the neck
Mechansism of action of metoclopramide?
Effects on emptying and LES?
Metoclopramide is a dopamine antagonist that causes increased gastric motility and increased lower esophageal sphincter tone.
Compare and Contrast the Haldane Effect vs. Bohr Effect
The Bohr and Haldane effects are complementary principles that help explain the efficient delivery of O2 to the tissue and CO2 to the lungs.
The Bohr effect states that in areas of high CO2, the hemoglobin has less affinity for O2 which is more readily released for consumption by the tissues.
The Haldane effect states that deoxygenated hemoglobin has a higher affinity for CO2. Once the deoxygenated hemoglobin reaches the lungs, the high O2 concentration decreases this affinity and the CO2 is released.

High grade stenosis of PDA (Posterior descending artery) would result in what phenomenon?
AV nodal blockade
What sugical OR device prevents:
Microshocks?
Macroshocks?
The equipment ground wire is the most reliable means to prevent microshock.
The ground fault interrupter* can prevent *macroshocks, but does not reliably prevent microshocks
3 causes of AutoPEEP
There are three main causes of auto-PEEP:
- Dynamic hyperinflation with intrinsic expiratory flow limitation: this occurs in COPD patients. COPD results in loss of elements that keep the lungs open during expiration. During exhalation, the closure of the airways results in air trapping. Applying external PEEP can help relieve this type of auto-PEEP.
- Dynamic hyperinflation without expiratory flow limitation: even when the airways are widely patent at the end of exhalation, if the volume delivered is too high, the exhalation time too short, or if exhalation is impeded by a blockage external to the patient (such as with blocked ETT or stuck exhalation valve) then air can get trapped. This is typically seen during rapid breathing patterns, use of high tidal volumes, when inspiration is greater than expiration, or when there is added flow resistance due to small endotracheal tubes. Removing the blockage, decreasing volume, or increasing exhalation time will help alleviate the problem.
- Exaggerated expiratory activity without dynamic hyperinflation: when strong expiratory muscle activity contributes to alveolar pressure at the end of the expiratory cycle there will be an end-expiratory gradient of alveolar to central airway pressure. This results in auto-PEEP phenomena even with low lung volumes.
From greatest to least, the vapor pressures of the volatile anesthetics are?
From greatest to least, the vapor pressures of the volatile anesthetics are: desflurane > isoflurane > sevoflurane.
What drug can treat Nausea and vomiting associated with high (T5) neuraxial block?
Nausea and vomiting may be associated with neuraxial block in up to 20% of patients and atropine is almost universally effective in treating the nausea associated with high (T5) neuraxial anesthesia.
Explanation: Unopposed parasympathetic (vagal) activity after sympathetic blockade causes increased peristalsis of the gastrointestinal tract, which can lead to nausea and is the primary mechanism behind nausea after spinal blockade. Atropine is an anticholinergic medication, thus is useful for treating nausea after high spinal blockade.
You are performing a TURP.
What nerve needs to be blocked OR use paralytic so this doesn’t happen to twitch?
Transurethral surgery of the bladder usually can be performed with spinal anesthesia without further consideration. If the tumor is along the lateral aspect of the bladder then the obturator nerve may be stimulated and the jerk reflex initiated even under spinal anesthesia. An obturator nerve block should be performed to help prevent this. Alternatively, general anesthesia with muscle relaxation can also be performed to prevent the reflex. If neuromuscular blocking agents are not used during general anesthesia, the obturator nerve block will still need to be performed.
What blood products does cryoprecipitate contain?
Cryoprecipitate contains vWF, fibrinogen, fibronectin, factor VIII, and factor XIII.


