Angiogenesis and Hypoxia in Tumors | Anti-angiogenesis strategies Flashcards
What is Angiogenesis
- Process by which new blood vessels are formed - Highly regulated - angiogenesis in adults usually occurs during reproduction, menstrual cycle, wound healing - Disease assocuated angiogenesis termed ‘neovascularisation’

What is VEGF (VEGF-A)
- Secreted PP which binds to cell surface R - Mitogen, chemoattractant and survival factor for endothelial cells - Loss of 1 copy of the VEGF gene causes aberrant BV formation - VEGF KO prevents embryonic angiogenesis
Discuss the organotypic co-culture model of angiogenesis
- Interaction of human dermal fibroblasts with human endothelial cells which results in the formation of tubules with lumens that are embedded in naturally produced ECM and closely resemble the capillaries formed during angiogensis
- Dependant on ANGIOGENIC FACTORS (bFGF and VEGF)
- Vessel ID by CD31 or vWF staining.
- Brack points and tube length can be quantified
- Figure: I.e; the greater the application of angiogenic factor (VEGF), the more angiogenic capillary formation. When you apply a drug (avastin - anti-VEGF therapy) then you start losing this VEGF angiogenic effect

VEGF R and L
- VEGF (L) Largely bind to VEGFR 2 (and to some extent VEGFR3)
- NRP1/2 are co-R which bind to VEGF

VEGF-A signalling via VEGFR2/KDR
- Transphosphorylation of Tyr residues which recruits modular domain proteins (PTB, SH2 domains)
- PLC gamma recruits IP3 which long term results in the regulation of vascular tone, angiogenesis, arterioprotection through the productuon of Nitric oxide (NO)
- LEARN THIS KEY DIAGRAM

Different mechanisms of BV formation
A) Sprouting Angiogenesis
B) Vasculogenesis
C) Intussusception
D) Vessel co-option
E) Vascular mimicry - TC take on the identity of endothelial cells
F) Tumor cell → EC differentiation: Cancer SC in the tumor can differentiate into multiple vasculature and different tumors.
Vessel formation by Angiogenic Sprouting
A) Selection of tip cells
- VEGF activation of tip cells via VEGFR2
- Suppression of tip cell phenotype in neighbouring cells via DII4/Notch ensures that the cell is only maximising growth from one tip
B) Stalk elongation and Tip Guidance
- Tip cell guidance along VEGF gradient
- Stalk elongation
- Lumen formation
- Pericyte recruitment by endothelial-derived PDGF-BB
C) Sprout formation, maturation, perfusion by the filopodia of the endothelial cells

Tumorigenesis and the angiogemic switch
1) somatic mutation
2) Small avascular tumor
3) Tumor secretion of angiogenic factors stimulates angiogensis
4) Rapid tumor growth and metastasis
- Formation of new vessels from pre-existing vasculature
- required for tumor growth (greater than 1mm^3) and metastasis
Mechanism:
1) Secretion of angiogenic factors
2) Proteolytic destruction of ECM
3) Endothelial cell proliferation and migration
4) Appearance of new tumor vasculature
5) Intravasation

Examples of pro/anti-angiogenic factors:
Pro: VEGF, FGF, PDGF
Anti: Angiostatin, Endostatin, Thrombospondin
Differences between tumor vascularisation and normal vascularisation
- Extrmely high endothelial proliferation rate: (Endothelial 3-13 v TC 47-2000 day)
- Distorted and disorganised architecture with sluggish blood flow, shunts and dead ends
- Leaky vessels
- Freq results in high interstitial fluid pressure and regions of hypoxia within the tumor
- abnormalities reduce the delivery of, and response to, conventional therapies

Importance of tumor hypoxia
- solid tumors contain v low regions of oxygen conc (hypoxia)
- hypoxia induces production of VEGF and other angiogenic cytokines
- hypoxia is associated with more metastatic phenotype, making it a prognostic determinant of cancer progression and therapeutic response.
- new hypoxia-selective therapies are being developed (e.g targeting of HIF1∂ TF. Still in preclinical or early clinical development)
The cellular oxygen-sensing system as a target for cancer drug therapy
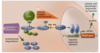
Advantages of Vessel vs Tumor cell targeting
- Rapidly dividing
- Accessibility: Easier to get a drug to vessels than to cells
- 1 capillary supports many tumor cells
- no drug resistance: vessel cells are more genetically normal
- applicable therapeutics to many solid tumors
Most clinically advanced therapeutics for Blood vessel targeting:
a) Vascular disrupting agents (VDAs)
b) Small molecule inhibitors
c) Antibodies
Vascular Disrupting agents - combretastin
- tubulin binding agent/colchicine binding site
- targets established tumor vessels
- selectively inhibits tumor blood flow
- destroys all but tumor rim, which continues to grow: vasculature around the tumor is a lot more normal which makes this drug a problem as you cannot get of all the tumor - you will get relapse. The advantage of this is that you can use combination therapy
Examples of tubulin binding VDAs in Clinical Trials

Examples of Combination therapies with VDAs

Mechanisms of action of small molecule inhibitors and monoclonal antibodies

Advantages and Limitations of Small molecule therapeutics vs Humanised Monoclonal Antibodies

Small molecule inhibitor (Vandetanib)
- Used in NSCLC
- Inhibitor of VEGFR
- result is a reduction in endothelial cell migration; vascular permeability; endothelial cell proliferation; lymphanogenesis etc
- Note in clinical trials the reduction in major vessels and capillaries supplying the tumor.

Anti-angiogenesis Antibodies (Avastin/Bevacizumab)
- Antibody to VEGF (Avastin/Bevacizumab)
- Blocks GF function and signalling
- First anti-angiogenesis strategy to be licensed by the FDA to treat human cancer
- Demonstrates increased OS survival in colorectal cancer
- Recently withdrawn for use in Breast cancer due to severe CV toxicity

Problems with Avastin/Bevacizumab
AE: Hypertension, increased incidence of thromoboembolic events (stroke, MI, haemmorhage etc)
Drug resistance: Probably due to the role of other angiogenic factors and reduced dependance on VEGF for tumor vascularisation
Dose selection: Too high a dose may prune vasculature too much, thus reducing the efficacy of chemotherapeutic drugs
What is the impact of altering VEGF levels and activity on the adult human vasculature?
- VEGF increases blood flow and reduces BP in animal models of peripheral and cardiac ischemic disease
- VEGF stimulates endothelial production of NO* and prostacyclin
- VEGF also probably required for maintenance of microvasculature in many tissues and for adult function in some organs
- Treatment of patients with cancr and eye disease with VEGF-targeted anti-angiogenic drugs have adverse impact on CV disease (Hypertension)

Resistance to Anti-Angiogenic therapy
Avastin/Bevacizumab increases period of PFS but has had little impact on the OS



