Aldehydes And Ketones Flashcards
What group do aldehydes and ketones contain?
Carbonyl group
Aldehydes and ketones
•Both contain a carbonyl group •Carbonyl group in aldehyde has a hydrogen atom attached- the aldehyde functional group occurs at the end of the carbon chain •In ketones- carbonyl group is located between two of the carbon atoms within the chain
Carbonyl functional group
.

Acetylaldehyde(ethanol)
.

Propionaldehyde(propanal)
.

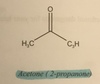
Acetone( 2-propanone)
.
Cinnamaldehyde
.

Vanillin
.

Formaldehyde
.

benzaldehyde
.

Nucleophillic Reaction of propanal
.

Nucleophillic reaction of acetone
.

Idoform Test for methyl Ketone
.

fellings solution reaction

Tollens reagent in silver mirror test

Names of aldehydes end in
Al
Names of ketones end in
One
Bonding in the carbonyl group
•Oxygen In carbon to oxygen bond far more electronegative •One of the two pairs of electrons that make up carbon to oxygen double bond even more easily pulled towards the oxygen- double bond very polar
One Physical property of aldehydes and ketones
Sharp odor
Most important reaction of ketones and aldehydes
Nucleophillic addition reaction
Nucleophillic addition reaction
The addition of a nucleophile to an electrophillic acceptor
Nucleophile forms new bond to carbonyl group carbon, carbon oxygen double bond breaks, proton bonds to oxygen
Carbonyl group undergoes addition reactions
Nucleophillic addition reaction of propanal
.

Nucleophillic addition reaction of acetone
.

Electrophile
Electrophile is the carbonyl group carbon of the ketone or aldehyde


