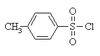
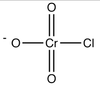
Alcohol Flashcards
What only type of alcohol react rapidly with halogen under normal condition (25 celcius)? What mechanism?
low weight, water soluble tertiary alcohol
SN1
What type of alcohol reacts smoothly with halogen to form haloalkene? Under what conditions and mechanism?
1º alcohol
heat reflux with water
SN2
Boiling point of alcohol in order (1º, 2º, 3º)
Why?
1º > 2º > 3º
the less hinder steric structure, the easier to have intermolecular H-bonding -> the higher boiling point
Solubility of alcohol (1 OH group, 2 OH group, 3 OH group) in water in order?
The more OH group, the more soluble in water
The ease of rxn of alcohol w/ halogen? (1º, 2º, 3º)
1º < 2º < 3º
What type of alcohol are not useful to make haloalkene? Why?
1º with ß branching and 2º
because they produce racemic products
What type of alcohol reacts with PBr3? Mechanism? Conditions?
1º 2º
SN2
at Oº C
Under what conditions does the water insoluble tertiary alcohol reacts with halogen? What mechanism?
at O° C and in either ether or THF solvents
SN1
What solvents are used in rxn btw alcohol & SOCl2?
pyridine/ 3º amine
What type of alcohol reacts with SOCl2? Mechanism?
1º and 2º
SN2
Ease of acid catalyzed dehydration of alcohol in order (1º, 2º, 3º)?
1º < 2º < 3º
What compound is this? What is it character? What product it can help alcohol to make?
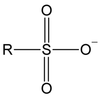
weak base, stable anion & good leaving group
haloalkene
What mechanisms for 2º & 3º alcohol react with halogen?
SN1
What type of alcohol react with H2CrO4 to give ketone?
What solvent?
2º alcohol
acetone
What type of alcohol react with PCC to give ketone?
What solvent?
2º alcohol
CH2Cl2