Acid base balance Flashcards
(37 cards)
Precise H+ regulation is essential because the activities of almost all ???? systems in the body are influenced by H+ concentration. Therefore, changes in H+ concentration alter virtually all ??????
Hydrogen levels are generally extremelly low. For example, the concentration of sodium in extracellular fluid (142 mEq/L) is about 3.5 million times as great as the normal concentration of H+, which averages only 0.00004 mEq/L. the normal variation in H+ concentration in extracellular fluid is only about one millionth as great as the normal variation in sodium ion (Na+) concentration
Precise H+ regulation is essential because the activities of almost all enzyme systems in the body are influenced by H+ concentration. Therefore, changes in H+ concentration alter virtually cell and body functions
Hydrogen levels are generally extremelly low. For example, the concentration of sodium in extracellular fluid (142 mEq/L) is about 3.5 million times as great as the normal concentration of H+, which averages only 0.00004 mEq/L. he normal variation in H+ concentration in extracellular fluid is only about one millionth as great as the normal variation in sodium ion (Na+) concentration
Study the pH levels of various body compartments.

There are various threats to normal acid/base balance:
Addition of acids or alkalis:
- Generation of ??? from ?????, which effectively behaves as an acid, as it dissociates into hydrogen ions
- Generation of nonvolatile/fixed acids from metabolism of ????
- metabolism of certain amino acids can create an acid load (such as ?- name 4), whilst others generate an alkali load (such as? - name 2)
- protein rich western diet is ???? loads
- Incomplete respiration
- ????during diabetes or starvation
- ????? due to inadequete tissue perfusion
- Loss of ????? in stool (diarrhoea), or loss of ??? in vomiting
There are various threats to normal acid/base balance:
Addition of acids or alkalis:
- Generation of Carbon dioxide from aerobic respiration , which effectively behaves as an acid, as it dissociates into hydrogen ions
- Generation of nonvolatile/fixed acids from metabolism of food
- metabolism of certain amino acids can create an acid load (such as arginine, lysine, methionine, cysteine), whilst others generate an alkali load (such as glutamate, aspartate)
- protein rich western diet is acid loads
- Incomplete respiration
- ketoacidosis during diabetes or starvation
- lacto-acidosis due to inadequete tissue perfusion
- Loss of alkali in stool (diarrhoea), or loss of acid in vomiting
There are 3 ways in which the body protects against changes in hydrogen ion concentration. Describe them
1) the chemical acid-base buffer systems of the body fluids, which immediately combine with an acid or a base to prevent excessive changes in H+ concentration; (2) the respiratory center, which regulates the removal of CO2 (and, therefore, H2CO3) from the extracellular fluid; and (3) the kidneys, which can excrete either acid or alkaline urine, thereby readjusting the extracellular fluid H+ concentration toward normal during acidosis or alkalosis.They do this by regulating the absorption and secretion of both bicarbonate and hydrogen.
Although acid/base balance is often transiently out of balance, these mechanisms work very quickly to correct this. It is important to note, that in order to maintain H ions, then it will be at the expense of something else (i.e bicarbonate, carbon dioxide) . It is therefore CRITICAL to appreicate the Hydrogen levels can be normal in the presence of acid-base disturbance.
When there is a change in H+ concentration, the ????? systems of the body fluids react within ???? to minimize these changes. ????? systems do not eliminate H+ from or add H+ to the body but only keep them tied up until balance can be re-established.
The second line of defense, the ????? system, acts within a few minutes to eliminate ???? and, therefore, H2CO3 from the body.
These first two lines of defense keep the H+ concentration from changing too much until the more slowly responding third line of defense, the ????, can eliminate the excess acid or base from the body. Although the ???? are relatively slow to respond compared with the other defenses, over a period of hours to several days, they are by far the most powerful of the acid-base regulatory systems.
When there is a change in H+ concentration, the buffer systems of the body fluids react within seconds to minimize these changes. Buffer systems do not eliminate H+ from or add H+ to the body but only keep them tied up until balance can be re-established.
The second line of defense, the respiratory system, acts within a few minutes to eliminate CO2 and, therefore, H2CO3 from the body.
These first two lines of defense keep the H+ concentration from changing too much until the more slowly responding third line of defense, the kidney, can eliminate the excess acid or base from the body. Although the kidneys are relatively slow to respond compared with the other defenses, over a period of hours to several days, they are by far the most powerful of the acid-base regulatory systems.
A buffer is simply a mixture of a weak ??? and its conjugate base or a weak base and its conjugate ???
The primary buffering system in the body is the Carbon dioxide - bicarbonate buffer system.
When a strong acid such as HCl is added to the bicarbonate buffer solution, the increased H+ released from the acid (HCl → H+ + Cl−) is buffered by HCO3-, driving the production of ???? and thus ??? + ???.
Thus, decreases by an amount that is equal to the H+ it consumes, and an equal amount of ??? is produced in the process.
A buffer is simply a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid
The primary buffering system in the body is the Carbon dioxide - bicarbonate buffer system.
When a strong acid such as HCl is added to the bicarbonate buffer solution, the increased H+ released from the acid (HCl → H+ + Cl−) is buffered by HCO3-, driving the production of carbonic acid and thus carbon dioxide + water.
Thus, decreases by an amount that is equal to the H+ it consumes, and an equal amount of CO2 is produced in the process.
The formula is very important. You would have carbon dioxide, bicarbonate and hydrogen in arterial blood gas. If the pH is low and, CO2 is high like when a patient isn’t breathing (forgetting compesnatoin), you have respiratory acidosis. If you have hydrogen ion excess (or bicarbonate), but normal CO2, then you have a metabolic acidosis.
Neither of these are taking into account compensation, or the possibility of mixed disorders.
Explain the difference between volatile and non-volatile acids.
Carbon dioxide is a volatile acid - meaning it can be eliminated from the body as a gas. Dietary acids are produced by anaerboc respiration are fixed (non-volatile) and cannot be converted to Co2.
The kidneys regulate acid-base balance in one of two ways. Firstly the reabsorb ???, and also secrete ????.
Secondandly the secrete “fixed” acid, using the ???? system and the ???? system.
The kidneys regulate acid-base balance in one of two ways. Firstly the reabsorb bicarbonate, and also secrete hydrogen.
Secondandly the secrete “fixed” acid, using the ???? system and the ???? system
Hydrogen ion secretion and HCO3− reabsorption occur in virtually all parts of the tubules except the ??? and ??? of the ????. Keep in mind that for each HCO3− reabsorbed, a H+ must be secreted.
About 80 to 90 percent of the HCO3− reabsorption (and H+ secretion) occurs in the ????? tubule, so only a small amount of HCO3− flows into the ???? tubules and ?????. In the thick ascending loop of Henle, another 10 percent of the filtered HCO3− is reabsorbed, and the remainder of the reabsorption takes place in the distal tubules and collecting ducts. As discussed previously, the mechanism by which HCO3− is reabsorbed also involves tubular secretion of H+, but different tubular segments accomplish this task differently.
Inability to reabsorb filtered HCO3 is a cause of ?????? acidosis.
Hydrogen ion secretion and HCO3− reabsorption occur in virtually all parts of the tubules except the thin descending and ascending portions of the loop of henle. Keep in mind that for each HCO3− reabsorbed, a H+ must be secreted.
About 80 to 90 percent of the HCO3− reabsorption (and H+ secretion) occurs in the proximal tubule, so only a small amount of HCO3− flows into the distal tubules and collecting ducts. In the thick ascending loop of Henle, another 10 percent of the filtered HCO3− is reabsorbed, and the remainder of the reabsorption takes place in the distal tubules and collecting ducts. As discussed previously, the mechanism by which HCO3− is reabsorbed also involves tubular secretion of H+, but different tubular segments accomplish this task differently.
Inability to reabsorb filtered HCO3 is a cause of metabolic acidosis.
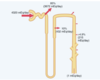
So, generally speaking we must ask how is bicarbonate reabsorbed from the tubular filtrate, bearing in mind it is doing so against a concentration gradient.
The epithelial cells of the primarily the ???? tubule, the thick segment of the ascending loop of Henle, and the early distal tubule all secrete H+ into the tubular fluid by ???? counter-transport. The energy for H+ secretion against a concentration gradient is derived from the ???? gradient favoring ???? movement ???? the cell. This gradient is established by the ????? pump in the basolateral membrane.
The secretory process begins when???? either diffuses into the tubular cells or is formed by metabolism in the tubular epithelial cells. ???, under the influence of the enzyme carbonic anhydrase, combines with H2O to form???, which dissociates into ??? and ???. The H+ is secreted from the cell into the tubular lumen by ????? counter-transport. The ??? moves into the cell down a concentration gradient that has been established by the ?????ATPase pump in the basolateral membrane. The gradient for Na+ movement into the cell then provides the energy for moving ??? in the opposite direction from the interior of the cell to the ?????.
The HCO3− generated in the cell (when H+ dissociates from H2CO3) then moves downhill across the ????? membrane into the renal interstitial fluid and the peritubular capillary blood. The net result is that for every H+ secreted into the tubular lumen, an HCO3− enters the blood. The reabsorption of filtered HCO3− does not result in net secretion of H+ because the secreted H+ combines with the filtered HCO3− and is therefore not excreted.
Transport of HCO3 across the basolateral membrane is facilitated by two mechanisms: (1) ?????− co-transport in the proximal tubules and (2) ????− exchange in the late segments of the proximal tubule, the thick ascending loop of Henle, and the collecting tubules and ducts.
So, generally speaking we must ask how is bicarbonate reabsorbed from the tubular filtrate, bearing in mind it is doing so against a concentration gradient.
The epithelial cells of the primarily the proximal tubule, and to a lesser extent the thick segment of the ascending loop of Henle, and the early distal tubule all secrete H+ into the tubular fluid by sodium-hydrogen counter-transport. The energy for H+ secretion against a concentration gradient is derived from the soidum concentration gradient favoring Sodium movement into the cell. This gradient is established by the Sodium/Potassium ATPase pump in the basolateral membrane.
The secretory process begins when CO2 either diffuses into the tubular cells or is formed by metabolism in the tubular epithelial cells.CO2, under the influence of the enzyme carbonic anhydrase, combines with H2O to form Carbonic acid, which dissociates into H+ and Bicarbonate. The H+ is secreted from the cell into the tubular lumen by sodium-hydrogen counter-transport. The sodium moves into the cell down a concentration gradient that has been established by the Sodium/potassium ATPase pump in the basolateral membrane. The gradient for Na+ movement into the cell then provides the energy for moving hydrogen in the opposite direction from the interior of the cell to the tubular lumen.
The HCO3− generated in the cell (when H+ dissociates from H2CO3) then moves downhill across the basolateral membrane into the renal interstitial fluid and the peritubular capillary blood. The net result is that for every H+ secreted into the tubular lumen, an HCO3− enters the blood.The reabsorption of filtered HCO3− does not result in net secretion of H+ because the secreted H+ combines with the filtered HCO3− and is therefore not excreted.
Transport of HCO3 across the basolateral membrane is facilitated by two mechanisms: (1) Na+-HCO3− co-transport in the proximal tubules and (2) Cl−-HCO3− exchange in the late segments of the proximal tubule, the thick ascending loop of Henle, and the collecting tubules and ducts.
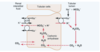
When there is an excess of ??? over ???in the urine, as occurs in metabolic alkalosis, the excess ???? cannot be reabsorbed; therefore, the excess ??? is left in the tubules and eventually excreted into the urine, which helps correct the metabolic alkalosis.
In acidosis, there is excess ??? relative to ????, causing complete reabsorption of the ????; the excess ???? passes into the urine in combination with urinary buffers, especially ???? and ?????, and eventually is excreted as salts. Thus, the basic mechanism by which the kidneys correct either acidosis or alkalosis is incomplete titration of H+ against HCO3−, leaving one or the other to pass into the urine and be removed from the extracellular fluid.
When there is an excess of bicarbonate over hydrogen in the urine, as occurs in metabolic alkalosis, the excess bicarbonate cannot be reabsorbed; therefore, the excess bicarbonate is left in the tubules and eventually excreted into the urine, which helps correct the metabolic alkalosis.
In acidosis, there is excess hydrogen relative to bicarbonate, causing complete reabsorption of the bicarbonate; the excess hydrogen passes into the urine in combination with urinary buffers, especially phosphate and ammonia, and eventually is excreted as salts. Thus, the basic mechanism by which the kidneys correct either acidosis or alkalosis is incomplete titration of H+ against HCO3−, leaving one or the other to pass into the urine and be removed from the extracellular fluid.
When H+ is secreted in excess of the HCO3− filtered into the tubular fluid, only a small part of the excess H+ can be excreted in the ionic form (H+) in the urine. This is because the minimal urine pH is about ???, corresponding to an H+ concentration of 10−4.5 mEq/L, or 0.03 mEq/L. Thus, for each liter of urine formed, a maximum of only about 0.03 mEq of free H+ can be excreted. To excrete the 80 mEq of nonvolatile acid formed by metabolism each day, about 2667 liters of urine would have to be excreted if the H+ remained free in solution.
The excretion of large amounts of H+ (on occasion as much as 500 mEq/day) in the urine is accomplished primarily by combining the H+ with buffers in the tubular fluid. The most important buffers are ????? buffer and ????? buffer. Other weak buffer systems, such as ???? and ?????, are much less important.
When H+ is titrated in the tubular fluid with HCO3−, this leads to reabsorption of one HCO3− for each H+ secreted, as discussed earlier. However, when there is excess H+ in the tubular fluid, it combines with buffers other than HCO3−, and this leads to generation of new HCO3 (which we discuss in other cards later)− that can also enter the blood. Thus, when there is excess H+ in the extracellular fluid, the kidneys not only reabsorb all the filtered HCO3− but also generate new HCO3−, thereby helping to replenish the HCO3− lost from the extracellular fluid in acidosis.
When H+ is secreted in excess of the HCO3− filtered into the tubular fluid, only a small part of the excess H+ can be excreted in the ionic form (H+) in the urine. This is because the minimal urine pH is about 4.5, corresponding to an H+ concentration of 10−4.5 mEq/L, or 0.03 mEq/L. Thus, for each liter of urine formed, a maximum of only about 0.03 mEq of free H+ can be excreted. To excrete the 80 mEq of nonvolatile acid formed by metabolism each day, about 2667 liters of urine would have to be excreted if the H+ remained free in solution.
The excretion of large amounts of H+ (on occasion as much as 500 mEq/day) in the urine is accomplished primarily by combining the H+ with buffers in the tubular fluid. The most important buffers are phosphate buffer and ammonium buffer. Other weak buffer systems, such as urate and citrtate, are much less important.
When H+ is titrated in the tubular fluid with HCO3−, this leads to reabsorption of one HCO3− for each H+ secreted, as discussed earlier. However, when there is excess H+ in the tubular fluid, it combines with buffers other than HCO3−, and this leads to generation of new HCO3 (which we discuss in other cards later)− that can also enter the blood. Thus, when there is excess H+ in the extracellular fluid, the kidneys not only reabsorb all the filtered HCO3− but also generate new HCO3−, thereby helping to replenish the HCO3− lost from the extracellular fluid in acidosis.
Titration of phosphate in the urine is dependent on delivery of phosphate to the urine and is relatively fixed - as such it cannot really be regulated.
Conversley, Ammonium CAN be regulated, and is infact upregulated in ????.
Acidosis.
Let’s look at how hydrogen ions titrate phosphate, and how this system generates NEW bicarbonate.
The process of H+ secretion into the tubules is the same as described earlier, the ??????? exchanger. As long as there is excess HCO3− in the tubular fluid, most of the secreted H+ combines with HCO3−. However, once all the HCO3− has been reabsorbed and is no longer available to combine with H+, any excess H+ can combine with ????? and other tubular buffers. After the H+ combines with ??? to form ????, it can be excreted as a ????? salt (NaH2PO4), carrying with it the excess H+.
There is one important difference in this sequence of H+ excretion from that discussed previously. In this case, the HCO3− that is generated in the tubular cell and enters the peritubular blood represents a net gain of HCO3− by the blood, rather than merely a replacement of filtered HCO3−. Therefore, whenever an H+ secreted into the tubular lumen combines with a buffer other than HCO3−, the net effect is addition of a new ??? to the blood. This process demonstrates one of the mechanisms by which the kidneys are able to replenish the extracellular fluid stores of HCO3−.
The process of generation of new bicarbonate is very simple. Carbon dioxide and ??? react inside the cell, and rapidly dissociate into a hydrogen and bicarbonate ion. The bicarbonate moves into the ????, whilst the hydrogen combines with HPO4 in the ????.
The process of H+ secretion into the tubules is the same as described earlier, the sodium/hydrogen exchanger. As long as there is excess HCO3− in the tubular fluid, most of the secreted H+ combines with HCO3−. However, once all the HCO3− has been reabsorbed and is no longer available to combine with H+, any excess H+ can combine with phosphate and other tubular buffers. After the H+ combines with HPO4- to form H2Po4-, it can be excreted as a sodium salt (NaH2PO4), carrying with it the excess H+.
There is one important difference in this sequence of H+ excretion from that discussed previously. In this case, the HCO3− that is generated in the tubular cell and enters the peritubular blood represents a net gain of HCO3− by the blood, rather than merely a replacement of filtered HCO3−. Therefore, whenever an H+ secreted into the tubular lumen combines with a buffer other than HCO3−, the net effect is addition of a new bicarbonate to the blood. This process demonstrates one of the mechanisms by which the kidneys are able to replenish the extracellular fluid stores of HCO3−.
The process of generation of new bicarbonate is very simple. Carbon dioxide and water react inside the cell, and rapidly dissociate into a hydrogen and bicarbonate ion. The bicarbonate moves into the interstitium, whilst the hydrogen combines with H2PO4 in the lumen.
The ammonium buffering system is also extremely important in the the excretion of non-volatile acids (fixed acids).
????? ion is synthesized from ????, which comes mainly from the metabolism of amino acids in the ?????. The glutamine delivered to the kidneys is transported into the epithelial cells of the proximal tubules, thick ascending limb of the loop of Henle, and distal tubules. Once inside the cell, each molecule of glutamine is metabolized in a series of reactions to ultimately form two ????? and two ?????. The NH4+ is secreted into the tubular lumen by a counter-transport mechanism in exchange for ???, which is reabsorbed. The HCO3− is transported across the ???? membrane, along with the reabsorbed Na+, into the interstitial fluid and is taken up by the peritubular capillaries. Thus, for each molecule of glutamine metabolized in the proximal tubules, two ???? are secreted into the urine and two ???? are reabsorbed into the blood. The HCO3− generated by this process constitutes new HCO3−.
The ammonium buffering system is also extremely important in the the excretion of non-volatile acids (fixed acids). The following refers to what happens in the proximal convoluted tubule.
Ammonium (NH4) ion is synthesized from glutamine, which comes mainly from the metabolism of amino acids in the liver. The glutamine delivered to the kidneys is transported into the epithelial cells of the proximal tubules, thick ascending limb of the loop of Henle, and distal tubules. Once inside the cell, each molecule of glutamine is metabolized in a series of reactions to ultimately form two bicarbonate and two ammonium. The NH4+ is secreted into the tubular lumen by a counter-transport mechanism in exchange for sodium, which is reabsorbed. The HCO3− is transported across the basolateral membrane, along with the reabsorbed Na+, into the interstitial fluid and is taken up by the peritubular capillaries. Thus, for each molecule of glutamine metabolized in the proximal tubules, two NH4 are secreted into the urine and two bicarbonate are reabsorbed into the blood. The HCO3− generated by this process constitutes new HCO3−.
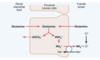
In the collecting tubules, the addition of NH4+ to the tubular fluids occurs through a different mechanism. Here, ??? is secreted by the tubular membrane into the lumen, where it combines with ??? to form ????, which is then excreted. The collecting ducts are permeable to ????, which can easily diffuse into the tubular lumen. However, the luminal membrane of this part of the tubules is much less permeable to ????; therefore, once the ??? has reacted with ??? to form ????, the ???? is trapped in the tubular lumen and eliminated in the urine. For each ???? excreted, a new ?????− is generated and added to the blood.
In the collecting tubules, the addition of NH4+ to the tubular fluids occurs through a different mechanism. Here, hydrogen is secreted by the tubular membrane into the lumen, where it combines with NH3 to form NH4, which is then excreted. The collecting ducts are permeable to NH3, which can easily diffuse into the tubular lumen. However, the luminal membrane of this part of the tubules is much less permeable to NH4; therefore, once the NH3 has reacted with H to form NH3, the NH4 is trapped in the tubular lumen and eliminated in the urine. For each NH4 excreted, a new HCO3 − is generated and added to the blood.
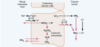
In all types of acid base disorder, there is a primary disturbance which tends to make H abnormal. However, as mentioned before the changes in H are often very transient. As such the disorder will often be reflected in abnormal levels of other substances, such as ? or ?. There are effectively two ways that our body responds to acid base/disorders.
- Given time, the kidneys will respond or compensate to this, either by ??? or by generating ???? and excrete ???.
- The lungs an also blow off carbon dioxide (or not as the case may be)
In all types of acid base disorder, there is a primary disturbance which tends to make H abnormal. However, as mentioned before the changes in H are often very transient. As such the disorder will often be reflected in abnormal levels of other substances, such as carbon dioxide or bicarbonate. There are effectively two ways that our body responds to acid base/disorders.
- Given time, the kidneys will respond or compensate to this, either by ??? or by generating ???? and excrete ???.
- The lungs an also blow off carbon dioxide (or not as the case may be)
The kidneys correct acidosis by increasing hydrogen ion secretion and increasing bicarbonate ion reabsorption.
Respiratory and metabolic acidosis both cause a decrease in the ratio of ???− to ????+ in the renal tubular fluid. As a result, there is excess H+ in the renal tubules, causing complete reabsorption of ????− and still leaving additional H+ available to combine with the urinary buffers ??? and ????. Thus, in acidosis, the kidneys reabsorb all the filtered ????− and contribute new ????− through the formation of ????+ and titratable acid.
In metabolic acidosis, an excess of H+ over HCO3− occurs in the tubular fluid primarily because of decreased ?????? of HCO3−. This decreased ????? of HCO3− is caused mainly by a decrease in the extracellular fluid concentration of HCO3−.
In respiratory acidosis, the excess ??? in the tubular fluid is due mainly to the rise in extracellular fluid ???, which stimulates H+ secretion.
As discussed previously, with chronic acidosis, regardless of whether it is respiratory or metabolic, there is an increase in the production of ???, which further contributes to the excretion of H+ and the addition of new HCO3− to the extracellular fluid. With severe chronic acidosis, as much as 500 mEq/day of H+ can be excreted in the urine, mainly in the form of NH4+; this excretion, in turn, contributes up to 500 mEq/day of new HCO3− that is added to the blood.
Thus, with chronic acidosis, increased secretion of ?? by the tubules has two effects; helps eliminate excess??? from the body and increases the quantity of ????− in the extracellular fluid. This process increases the HCO3− part of the bicarbonate buffer system which, in accordance with the Henderson-Hasselbalch equation, helps raise the extracellular pH and corrects the acidosis. If the acidosis is metabolically mediated, additional compensation by the lungs causes a reduction in Pco2, also helping to correct the acidosis.
The kidneys correct acidosis by increasing hydrogen ion secretion and increasing bicarbonate ion reabsorption.
Respiratory and metabolic acidosis both cause a decrease in the ratio of bicarbonate− to H+ in the renal tubular fluid. As a result, there is excess H+ in the renal tubules, causing complete reabsorption of bicarbonate− and still leaving additional H+ available to combine with the urinary buffers phosphate and ammonium. Thus, in acidosis, the kidneys reabsorb all the filtered bicarbonate− and contribute new bicarbonate− through the formation of Ammonium and titratable acid.
In metabolic acidosis, an excess of H+ over HCO3− occurs in the tubular fluid primarily because of decreased filtration of HCO3−. This decreased filtration of HCO3− is caused mainly by a decrease in the extracellular fluid concentration of HCO3−.
In respiratory acidosis, the excess H+ in the tubular fluid is due mainly to the rise in extracellular fluid CO2, which stimulates H+ secretion.
As discussed previously, with chronic acidosis, regardless of whether it is respiratory or metabolic, there is an increase in the production of NH4, which further contributes to the excretion of H+ and the addition of new HCO3− to the extracellular fluid. With severe chronic acidosis, as much as 500 mEq/day of H+ can be excreted in the urine, mainly in the form of NH4+; this excretion, in turn, contributes up to 500 mEq/day of new HCO3− that is added to the blood.
Thus, with chronic acidosis, increased secretion of H+ by the tubules has two effects; obviously, helps eliminate excess H+ from the body and also increases the quantity of bicarbonate− in the extracellular fluid. This process increases the HCO3− part of the bicarbonate buffer system which, in accordance with the Henderson-Hasselbalch equation, helps raise the extracellular pH and corrects the acidosis. If the acidosis is metabolically mediated, additional compensation by the lungs causes a reduction in Pco2, also helping to correct the acidosis.
Describe the primary and secondary (compensatory) changes you would expect to see in respiratory and metabolic acid/base disorders.

What information/tests do you need to diagnose an acid/base disorder?
- Clinical history
- Blood gases - H+, bicarbonate, CO2
- Electrolytes - Sodium, Calcium, Potassium, Chloride
Outline the approach to a diagnosis of acid-base disorders.
There are various causes of metabolic acidosis, which is the most common acid/base disorder.
-
Addition of extra acid
- What can cause this?
- Failure to excrete acid
- What can cause this?
- Loss of bicarbonate
- What can cause this?
The primary abnormality you would see is a drop in ???.
The compensatory response is a ??? in ??? due to respiratory drive.
-
Addition of extra acid
- Generation of acid through metabolism - lacto-acidosis, keto-acidosis
- Ingestion of something which can be metabolised to form an acid - methanol
- Failure to excrete acid
- Renal tubular acidosis
- Loss of bicarbonate
- diarrhoea or again, renal tubular acidosis
The primary abnormality you would see is a drop in plasma bicarbonate
The compensatory response is a fall in CO2 due to respiratory drive.
Study causes of metabolic acidosis