Acid and Base Electrolytes Flashcards
Hyponatremia
- Spurious
- Pseudohyponatremia
- Hyperglycemia
- True hypotonic hyponatremia
Spurious
- blood is drawn proximal to an IV infusion or from a central venous line
Pseudohyponatremia
- May affect any instrument that utilizes “indirect” method of measurement in which sample is prediluted before analysis
- These analyzers calculate the plasma/serum sodium on assumption that water content of plasma is 93%
- The above assumption may be incorrect in the following in which water content in original sample is lower than 93%
- hypertriglyceridemia
- hypercholetersolemia
- hyperproteinemia
- Serum osmolarity is normal and an osmolal gap is present
- Direct potentiometry, as performed on blood gas analyzers, not affected
Hyperglycemia
- True physiologic shift in sodium ions into extracellular space, producing hyponatremia that is real but unrelated to an intrinsic defect in sodium homeostasis
- Hypertonic (>295 mOsm/kg) hyponatremia suggests marked hyperglycemia, but can be seen in mannitol administration
True Hypotonic Hyponatremia (<280 mOsm/kg)
Degree of change in sodium concentration attributable to glucose calculated by:
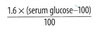
True hypotonic hyponatremia
< 280 mOsm/kg
-
Patient is hypovolemic
-
Renal losses
- suggested by increased urine sodium (UNa > 30 mmol/L)
- may be caused by
- diuretics
- renal medullary disease
- primary adrenal insufficiency (Addison disease)
- renal tubular acidosis Type I
- Cerebral salt wasting syndrome
- Extrarenal sodium losses
- GI tract (vomiting, diarrhea)
- 3rd spacing (peritonitis, pleuritis)
- Suggested by low urine sodium (UNa<30)
-
Renal losses
-
Patient is euvolemic
- SIADH
- Psychogenic polydipsia
- hypothyroidism
- primary adrenal insufficiency (Addison)
- drugs with ADH like effect (vasopressin)
- desmopressin
- SSRIs
- TCAs
- ecstasy
-
Patient is hypervolemic
- CHF
- cirrhosis
- nephrotic syndrome
Hypernatremia
- Most commonly seen in people with excess water loss and inability to respond to their thirst: infants, ICU, and debilitated
- extrarenal water loss:
- diarrhea
- vomiting
- burns
- Renal water loss
- osmotic diuretics
- loop diuretics
- postobstructive diuresis
- intrinsic medullary renal disease
- extrarenal water loss:
- May be iatrogenic: administration of sodium as part of IV fluids, Na HCO3, or other intervention
- Diabetes insipidus
- Central
- damage to the hypothalamus or neurohypopysis related to surgery
- Space occupying lesions in/near sella
- head trauma
- infiltrative lesions such as eosinophilic granuloma and sarcoidosis
- Nephrogenic
- Medullary diseases (sickle cell and tubulointerstitial nephritis)
- electrolyte disturbances (hypokalemia and hypercalcemia)
- renal tubular acidosis
- Fanconi syndrome
- drugs (lithium, demeclocycline, colchicine, amphotericin B, gentamicin, furosemide)
- Central
Hypokalemia
-
Renal loss
- Elevated urinary potassium (UK > 30 mEq/day)
- Diuretics
- hypomagnesemia
- antibiotics (carbenicillin, amphotericin B)
- mineralcorticoid excess
- renal tubular acidosis types I and II
- severe Cushing syndrome
- congenital adrenal hyperplasia
- Bartter syndrome
- Liddle syndrome
- Gitelmand syndrome
- licorice
- hyperreninism
- GI loss
- Urinary potassium is low (UK < 30 mEq/day)
- Vomiting
- NG tube suction
- diarrhea
- large villous adenoma
- Trancellular shift
- metabolic alkalosis or correction of diabetic ketoacidosis
- In diabetic ketoacidosis there is an initial hyperK (like most acidotic states), but correction of DKA results in profound hypokalemia unless supplemental potassium is given
Pseudohyperkalemia
- Pseudohyperkalemia
- elevated measured potassium in absence of in vivo hyperK
- Causes
- In vitro cellular leak of potassium from hemolysis
- in vitro clot formation (release of potassium from platelets especially in patients with hyperkalemia; serum has a higher potassium result than plasma for this reason)
- leukocytosis
- Prolonged tourniquet time
- excessive fist clenching
- traumatic draw
- inappropriate order of tubes drawn
- venipuncture proximal to IV infusion and small gauge needles
Hyperkalemia etiology
- acidosis
- nearly all cases of acidosis have hyperK, exceptions including RTA I and II in which potassium is low
- renal failure
- potassium sparing diuretics (SEAT)
- spironolactone
- triameterene
- amiloride
- eplelrenone
- adrenal insufficiency
- iatrogenic
- rhabdomyolysis
Calcium metabolism
- 50% of serum calcium is bound to protein, mainly albumin
- in hypoalbuminemia free (ionized) calcium is normal but total calcium is low
- acidosis decreases binding of calcium to albumin, increasing free calcium
- alkalosis decreases free calcium
Hypercalcemia presentation
- nephrolithiasis
- lethargy
- hyporeflexia
- slowed mentation
- nausea
- vomiting
- constipation
- depression
- high peaked T waves on ECG
- increased risk of pancreatitis
- increased risk of peptic ulcer disease
- long term hypercalcemia with concomitant hyperphosphatemia results in metastatic calcification of vessel walls and soft tissue (calciphylaxis)
Hypercalcemia etiology
- Primary hyperparathryoidism
- excess PTH results in
- increased serum calcium with decreased serum phosphate
- increased chloride/phosphate ratio
- increased urinary cAMP
- Causes:
- Parathyroid adenoma
- 4-gland hyperplasia
- parathyroid carcinoma
- excess PTH results in
- Secondary hyperparathyroidism
- excessive secretion of PTH in response to hypocalcemia of any cause (most often chronic renal failure)
- Tertiary hyperparathyroidism
- after long period of secondary hyperPTH, autonomous parathyroid function may develop
- post renal transplant
- Malignancy
- mostly from PTH related protein in SCC of lung, head and neck, skin, cervix, esophagus, breast
- T cell lymphoma
- small cell carcinoma of ovary
- paraganglioma
- renal cell carcinoma
- islet cell tumors
- multiple myeloma
- HCC
- Familial hypocalciuric hypercalcemia: CASR gene on 3q
- Drugs
- thiazides
- calcium-containing antacids or calcium supplements (milk-alkali syndrome)
- Hypervitaminosis D: increased calcium and phosphate
- Hyperthyroidism
- Addison
- acromegaly
- Sarcoidosis
- Addison
- Immobilized Paget patient
Assays for PTH hormone
- Sensitive to particular portions of the PTH molecule
- N terminal and intact PTH have biological activity and are rapidly cleared from the blood (t1/2 of 5 minutes)
- PTH-C and PTH-M have t1/2 of up to 36 hours
Forms of parathyroid hormone
- Intact PTH: biologically active, short half-life
- N-terminal PTH: biologically active, short half-life
- Mid-region PTH: not biologically active, long half-life
- C-terminal PTH: not biologically active, long half-life
Hypocalcemia presentation
- Neurologic excitability
- perioral tingling (parasthesia)
- muscle spasm
- hyperreflexia
- Chvostek sign
- Trousseau sign
- lengthened QT interval
- low voltage T waves
- dysrhythmias
- laryngeal spasm
- tetany
- respiratory arrest
Etiology of hypocalcemia
- Hypoproteinemia
- Low albumin, ionized calcium usually normal
- Chronic renal failure: hyperphosphatemia
-
Drugs (HAM LOG)
- heparin
- glucagon
- osmotic diuretics
- loop diuretics (e.g., furosemide)
- aminoglycosides
- mithramycin
-
Hypoparathyroidism:
- most often iatrogenic
- post-surgical
- hypomagnesemia (however, mild transient hypomagnesemia may increase PTH)
- DiGeorge syndrome
- autoimmune
- Medullary thyroid carcinoma: rarely affects serum Ca
- Hyperphosphatemia: calcium chelation
- Vitamin D deficiency: Most have normal calcium levels
- Pancreatitis: extensive calcium deposition
- Massive transfusion: citrate
Define:
Acidemia
Alkalemia
Acidemia: arterial pH < 7.35
Alkalemia: arterial pH > 7.45
Simple acid/base disorder
and
Complex acid/base disorder
- simple: primary acid base disturbance and associated compensation
- complex: more than one primary acid base disturbance
Henderson-Hasselbalch equation
pH = pKa + log(base/acid)
pH = 7.4 (normally)
pKa = 6.1
bicarbonate = 24 mol/L
acid (carbonic acid) = 0.03 x PaCO2 = 0.03 x 40 mmHg = 1.2 mol/L
Thus, pH = 6.1 + log(bicarb/[0.03 x PaCO2])
Classifying an acid base disorder
- Determine the primary abnormality
- Metabolic acidosis: bicarb decreased (< 25 mEq/L)
- Respiratory acidosis: pCO2 usually > 44 mmHg while pH decreases
- The above are opposite for alkalosis (metabolic alk: bicarb > 25 and for respiratory pCO2 < 40 mmHg)
- Determine if compensation is appropriate
- Differentials
How to determine if compensation in acid base disorder is appropriate
- Metabolic acidosis: for each 1.3 mEq fall in bicarb, the pCO2 should decrease by 1.0 mmHg
- Metabolic alkalosis: for each 0.6 mEq rise in bicarb, the pCO2 increase by 1 mmHg
- Respiratory alkaloses or acidosis
- Acute: for each 1 mmHg change in pCO2 the bicarb changes by 0.1 in the same direction
- Chronic: for each 1 mmHg change in pCO2 the bicarb changes by 0.4 in the same direction
Calculations and differential for metabolic acidosis
Characterized by presence or absence of anion gap and osmolal gap
- Calculate the anion gap:
- anion gap = [Na] - [Cl] - [HCO3]
- normal is <12
- In nonanion gap acidosis the chloride level is often elevated
- A low anion gap may be caused by hypoalbuminemia and paraproteinemia
- Calculate the osmolal gap:
- osmolal gap = osmmeasured - (2[Na] + [glucose]/18 + [BUN]/2.8)
- [Na] is in mEq/L, [glucose] is in mg/dL, and [BUN] is in mg/dL
- when international units are used:
- osmmeasured - 2[Na] - [glucose] - [BUN]
- Normal osmolal gap <10
- Increased anion gap (>=12)
- methanol
- uremia
- ketoacidosis (diabetes, EtOH, starvation)
- Paraldehyde
- Lactic acidosis
- ethylene glycol
- salicylate
- Normal anion gap (<12)
- diarrhea
- recovery phase diabetic ketoacidosis
- ureterosigmoidostomy
- NH4Cl
- Carbonic anhydrase inhibitors
- Total parenteral nutrition
- Renal tubular acidosis
- Increased osmolal gap
- with metabolic acidosis
- methanol
- propylene glycol
- paraldehyde
- ethanol (sometimes)
- without metabolic acidosis
- isopropanol
- glycerol
- sorbitol
- mannitol
- acetone
- ethanol (sometimes)
- with metabolic acidosis
Differential for metabolic alkalosis
Metabolic alkalosis: disorders characterized by chloride responsiveness or resistance
- Chloride responsive (UCl < 10)
- diuretic therapy
- vomiting
- nasogastric tube suction
- villous adenoma
- carbenicillin
- contraction alkalosis
- Chloride resistant (UCl > 10)
- Hyperaldosteronism
- Cushing syndrome
- Exogenous steroids
- Licorice
- Bartter syndrome
- Milk-alkali syndrome
Differential for respiratory acidosis
- impairment to ventilation
- airway obstruction
- alveolar infiltrates
- perfusion defects
- neuromuscular disease
Respiratory alkalosis differential
- hypoxemia in which compensatory hyperventilation leads to hypocapnia
- anxiety
- CNS insults
- pregnancy
- medications


