4.2- Alcohols, haloalkanes and analysis Flashcards
What is a primary Alcohol?
A primary alcohol has the alcohol group attached to the end carbon on a change
What is a Secondary alcohol?
It has the alcohol group attached to a carbon with two alkyl chains attached
What is a tertiary alcohol?
Has the alcohol group attached to a carbon atom with three alkyl chains attached
Define volatility
Volatility is how easily a substance evaporates
As alkyl chain length increases, the solubility of the alcohol _______
Decreases
Another name for dipole induced dipole is
London forces
In oxidation of a primary alcohol what are the reaction conditions?
Gentle heating
In oxidation of a primary alcohol what is the product formed with gentle heating?
Aldehyde
What is this?
-COOH
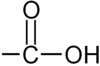
Carboxylic Acid
What is this?
-CHO
Aldehyde
What is this?
C-CO-C
Ketone


