4: Testing Materials_checked Flashcards
What are the properties of ceramics?
Hard - difficult to scratch
Brittle - easily shatter into pieces due to rigid structure/ cracks spread through them when they break
Stiff - difficult to stretch or bend due to strong bonds between atoms.
Describe structure of a ceramic.
The arrangement of atoms in a ceramics can be crystalline or poly-crystalline [where there are any regions or grains of crystalline structure. The atoms in each grain line up in the same direction.
Some ceramics like glass are amorphous - there is no pattern; the atoms are arranged at random. The quicker a molten a material is cooled, the more likely it is to be amorphous.
The random atomic bonding means there is no slips planes in ceramic lattices. They also don’t have dislocations which can move meaning that ceramic materials rarely plastically deform before they fracture. The rigid structure means that ceramics are very brittle materials.
The ceramics are either ionically or covalently bonded in a giant rigid structure. The strong bonds between atoms make ceramics stiff.
Ceramics being brittle is why cracks spread through them when they fracture, because the applied force acts over a very small area so the stress is high
Describe the properties of Metals
Some metals are malleable (can be shaped easily) and some are ductile (can be drawn into wires) this is due to the dislocations (missing atoms in metal structure) allowing planes (rows) of metal atoms to slip over each other when a force is applied
Some metals are stiff - strong metallic bonds between ionic lattice and delocalised electrons
Metals are good conductors - metals have a sea of delocalised electrons that allows metals to conduct electricity
Metals are tough: it absorbs a lot of energy (deforms plastically) per unit area before fracture. This is because when you apply a stress the metal deforms plastically in the region of the crack, making the crack broader reducing stress around it
Describe the structure of Metals
Metals consist of crystalline metallic lattice - atoms are arranged in a regular repeating pattern; surrounded by a sea of delocalised or free electrons
PRACTICAL Explain, when testing Young’s modulus, why the wire should be as thin and as long as possible?
List some safety procedures
The longer and thinner the wire, the more it extends for the same force. This reduces the uncertainty in length measurements
Safety goggles to be worn at all times due to risk of wire snapping and damaging eyes. Box to collect the masses when wire breaks and the masses drop: this helps to prevent masses falling on feet.
PIC: What happens when you apply a force to a metal with dislocations?
When a force is applied to a metal, the inter-atomic spacing between the ions increases. This increase is unform during elastic deformation. Once the stress is high enough to cause plastic deformation, the planes [sheets of metal ions] within the metal slip over each other. If there is a dislocation in the metal, the stress needed to cause slipping is lower than the stress needed to cause slipping in a perfect metal.

What happens when you put the atoms of a second metal in dislocations?
What is this process called?
What’s the effect?
Atoms of a second metal (impurities) can be placed inside dislocations to pin them down. This increases the stress needed to cause slipping.
This process is called alloying.
This causes metals to be harder and less ductile
What is a perfect metal?
A metal lattice with no dislocations (missing atoms) or impurities.
What is a polymer?
Name 2 types of polymers.
A polymer is a long molecular chain, made up of single repeating units called monomers
There are man made polymers (polythene) and natural polymers (rubber)
Give an example of a polymer
Rubber: sulfur atoms form cross links with the polymer chains; the more sulfur that is added, the more cross links that are formed and the stiffer the rubber [polymer] becomes.
PIC: Structure and bonding of a polymer
- The monomers in a chain are covalently bonded so they’re very hard to separate.
- Polymer chains are often entangled but can be unravelled by rotating about their bonds when you pull them. This makes polymers flexible.
- The strength and number of bonds between the chains also affects the polymer’s flexibility. if the cross link bonds (chains tied at regular interval) are stronger and you have more of them, the more rigid the polymer

What is Hooke’s law equation?
What is the constant a measure of?
For small extensions, the force, F, is proportional to the extension, x
F = kx
F = force, in Newtons
x = extensions, in metres
k = constant of proportionality (spring constant), Nm-1
The spring constant is a measure of stiffness
k is small when small force gives big extension
k is big when big force gives small extension
PIC: PRACTICAL: Force Extension graph for a rubber band - explain the process.
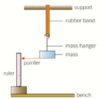
- Start with the shown apparatus,
- Use a stiff wire wrapped around the bottom of the mass to provide a pointer.
- Measure the unstretched length of the band
- Then measure the length of the band with each mass you add on find the extension by subtracting old length from new length.
- Remove each mass and record the extension
- Plot graph of Force on y-axis and extension on x-axis

What is material compression?
What is the force called when you stretch a material
Compression occures when you squash a material.
Stretching forces are also called tensile forces.
What is elastic deformation for a wire?
When you deform a wire but it returns to its original length when the force (stress) is removed
PIC: What is the elastic limit, E?
Up to the elastic limit, an object will elastically deform; beyond the elastic limit, it will plastically deform.

What is plastic deformation for a wire?
A wire that has been stretched beyond its elastic limit. The wire is permanently deformed and will not return to its original length when the stress (force) is removed
PIC: What is the fracture stress, B?
The stress at which the object breaks. It is the point after plastic deformation at which the object breaks. At this point, the stress becomes so great that atoms separate completely and the material [object] breaks.

PIC: What is the limit of proportionality, P?
The point before the elastic limit where the graph is straight and extension is proportional to the force
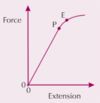
What happens when you stretch a material?
The material stores energy in the form of elastic strain energy.
State 2 equations to calculate energy stored in a spring
E = 1/2 Fx
E = 1/2 kx(x) => E = [1/2]kx²
PIC: What does the area under an extension force graph give when elastically deforming?
When a body deforms elastically the energy stored is equal to the energy transferred while stretching the spring.
The energy transferred while stretching a material can be found from the area under the line

What is the equation for stress? Units?
Stress is the force applied (tension) divided by cross sectional area (force per unit area)
stress, σ = tension / cross sectional area
Units: Nm-2 or Pa
PIC: What is the yield stress?
Yield stress is the stress at which a material begins to deform plastically and become permanently deformed

What is the equation for strain? Units?
Strain its the change in length divided by the original length
Strain, ε = extension / original length
No units as it’s a ratio of two lengths that cancel
What is stress dependent on?
What is strain dependent on?
Stress is dependant on the cross sectional area
Strain is dependant on original length
PIC: What is ultimate tensile strength, UTS?
UTS is the maximum stress that a material can withstand before breaking

PIC: Describe a stress strain graph for a brittle material.
The stress strain graph shows very little plastic deformation. The graph is linear for all its length.

What is the equation for Young’s Modulus? Units?
State an indication that you value of YM is correct
YM is a measure of stiffness
YM = stress / strain
Units: Nm-2 or Pa
YM is usually a very large value
PIC: PRACTICAL: finding young’s modulus how to determine young’s modulus for a metal wire using Searle’s apparatus
- The test wire should be as thin and as long as possible. The longer and thinner the wire the more it extends for the same force. This reduces uncertainty in extension measurements.
- Using a micrometer, measure the cross-sectional area at three different places along the length of the wire. Find an average, assuming the cross-section is circular.
- Clamp the wire to the bench, so that you can hang weights off one end of it. Start with the smallest weight to straighten the wire [this is not included in the calculations]
- Measure the distance between the fixed end of the wire and the marker - this is the unstretched length.
- Then if you increase the weight, the marker moves as the wire stretches.
- Increase the weight in steps e.g. 1N intervals, recording the extension each time. You can use a digital scales to determine the weight more accurately.
- Calculate stress, and strain values at each weight interval.
- Plot a graph of stress against strain.
- Identify the elastic region of the graph and determine the YM by calculating the gradient.

What happens to the relationship between force and extension after the elastic limit?
The material will stretch further for a given force. The material will deform plastically as the presence of dislocations allow planes of atoms to slip over one another.
Consequently, the relationship between force and extension after the elastic limit will no longer be proportional.

What is elastic strain energy?
The energy stored in a stretched material
E = ½ Fx
E = ½kx2

Describe a typical Stress Strain graphs for ductile materials…
Linear region where stress is propotional to strain. In this region, elastic deformation takes place [up to the proportional limit].
The material behaves elastically up to the elastic limit. Beyond, the elastic limit, stress is no longer proportional to strain. The stress-strain graph begins to curve as the material deforms plastically

Why is copper perfect for making electric wires?
Copper is ductile, and with its high electrical conductivity this means that it’s the ideal material for electric wires
On a stress strain graph for ductile material, what is the yield stress?
At the yield point the material suddenly starts to stretch without any extra load. the yield stress is the stress at which a large amount of plastic deformation takes place with a constant or reduced load

When does the graph of force against extension start to curve?
Starts to curve after the limit of proportionality.
Explain why the work done = [½]Fx
Work done = force * displacement
However, the force on the material isn’t constant.
It rises from zero up to force F. To calculate the work done, use the average force between zero and F,
Average force = [½]F
Therefore, average work = [½]Fx
Steel cables (on a crane) are strong. Describe one other mechanical property that is important
Steel cables are also stiff / have a high YM
This means steel cables do not stretch (too far] under stress.
Steel cables are tough [not brittle] ; so do not break easily. Consequently, cracks don’t propagate and the the cables do not snap easily
Why would plastic make a better plug casing than ceramics?
Plastics are tougher and less brittle than ceramics and therefore will be more durable as plug casing.
Define brittle.
Brittle materials undergoes little/no plastic deformation before fracture. Britt;e materials fracture before they reach their elastic limit.
Describe the term tough
Toughness is a measure of the energy (impact) a material can absorb before it breaks.
What is strength?
Describe strong materials
Strength is a measure of how much a material can resist being deformed (bent, stretched, fractured etc.) by a force without breaking.
Strong materials can withstand high stresses without deforming or breaking. This can be resisting a pulling force (tensile strength) or a squeezing force (compressive strength)
Describe the term stiff
Describe a stiff material
Stiffness is measured by the Young’s modulus – the higher the value, the stiffer the material.
Stiff materials are difficult to both bending and stretching.
Define ductile
Ductile materials can be drawn into wires without losing their strength.
Define hard
Hard materials resist indentation, cutting and abrasion
Define polycrystalline
Polycrystalline materials consist of a number of grains all oriented differently to one another. There is a regular structure within each grain e.g. the atoms in a particular grain line up in the same direction


