3.3.11 - Amines Flashcards
Name the aliphatic amine and state the type of amine it is


Name the aliphatic amine and state the type of amine it is


Name the aliphatic amine and state the type of amine it is


Name the aliphatic amine and state the type of amine it is


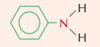
Name the aromatic amine and state the type of amine it is

Explain how a quaternary ammonium ion forms
Lone pair of electrons on nitrogen atom in tertiary amine can also bond with 4th organic group
Explain how quaternary ammonium salts form
Quaternary ammonium ions are positively charged ∴ form bonds around with -ve ions & form complexes (quaternary ammonium salts)
What type of quaternary ammonium salts are used as cationic surfactants?
Quaternary ammonium salts with at least 1 long hydrocarbon chain

What products are cationic surfactants used in?
Fabric cleaners (e.g. fabric conditioner) and hair products (e.g. conditioner)
Describe and explain why cationic surfactants are useful in fabric cleaners and hair products
- Hydrocarbon tail binds to non-polar substances like grease & cationic head dissolves in water
- Positively charged part (ammonium ion) will bind to negatively charged surfaces e.g. hair and fibre
- Gets rid of static
Why do amines act as (weak) bases?
∵ accept protons
Explain how amines can accept protons
Lone pair of electrons on nitrogen atom can form dative covalent (coordinate) bond with H+ ion
What does the strength of a base depend on (for amines)?
Depends on how available nitrogen’s lone pair of electrons is
More available the lone pair is = more likely amine is to accept proton & stronger base it’ll be
When is a lone pair of electrons more available?
When its electron density is high
Order the amines from the weakest to strongest base
Ammonia, aliphatic amines, aromatic amines
- Aromatic amines (weakest)
- Ammonia
- Aliphatic amines (strongest)

Explain why primary aromatic amines (e.g. phenylamine) act as very weak bases
- Benzene ring draws electrons towards itself and nitrogen lone pair gets partially delocalised onto the ring
- ∴ the electron density on nitrogen ↓, making lone pair much less available

Explain why primary aliphatic amines act as strong bases
- Alkyl groups push electrons onto attached groups
- ∴ electron density on nitrogen atom ↑
- Makes lone pair more available

Why are amines considered nucleophiles?
∵ have lone pair of electrons
Name the 2 mechanisms that amines partake in. Include the reactants.
- React with halogenoalkanes in nucleophilic substitution
- React with acyl chlorides and acid anhydrides in nucleophilic addition-elimination
Name 2 ways you can make aliphatic amines
- Heating halogenoalkane with excess ammonia
- Reducing a nitrile (to a primary amine)
Draw the mechanism to show how ethylamine can be made by reacting ammonia with bromoethane

When heating halogenoalkane with ammonia, what mixture of products do you get?
Mixture of primary, secondary, tertiary amines + quaternary ammonium salts
Explain how heating halogenoalkane with ammonia results in a mixture of primary, secondary, tertiary amines and quaternary ammonium salts
- Primary amine 1st produced has lone pair of electrons = nucleophile
- ∴ can react with any remaining halogenoalkane in nucleophilic substitution reaction
- Further substitutions take place as long as there’s remaining halogenoalkane
- Happens up to quaternary ammonium salt = can’t react further ∵ has no lone pair

Write an equation for when a primary amine acts as a nucleophile with CH2CH2Br

Mechanism is similar to reaction of ammonia + halogenoalkane: 2 amine molecules react with halogenoalkane in succession to form more substituted amine (e.g. primary amine forms secondary amine) & ammonium salt with similar structure to original amine














