1: Immunology of Tumor and Cancer Flashcards
What are the different evidences for tumor surveillance (überwachung) by the immune system?
- Immune system targets tumor with antibodies (e.g. CDR2) –> if antigens are present this might lead to auto-immune neurogenic disease
- autopsied of (otherwise healthy) adults show microscopic cancer colonies –> immune control to prevent further progression?
- Patients with melanoma were treated and disease free but when they donated organs the recipients got melonama–> “first patients developted a “immunity against melanoma”
- In immunosupression, the risk of cancer increased
- Women have in general stronger immune responses and are less likely to die from cancer
Wht is the principle of cancer immunotherapy?
Overall: the imune system controlls malignant cells
Immunotherapie tries to enhance the natural immune response to cancer cells
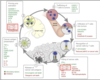
Explain the cancer immunuty cycle and how the immune system controlls malignant cells
- Cancer cell dies and releases cancer cell antigens
- They are sensed and presented by Antigen-presneting cells (normally Dendtritic cells)
- In lympth nodes: T cells get primed and activated
- Travel to tumor via the blood stream
- Infiltration of tumor with T cells (TILs)
- Cancer cells are recognised by T cells
- Killing of cancer cells (goes back to 1)

What are TIL?
Tumor-Infiltrating lymphocytes
–> lymphocytes that travel to tumors (+ recognise tumor antigens and might eventually kill them)
What is the proble with the immune control of cancer cells?
Cancer cells have a huge immune selection pressure –> might mutate further and make it not detectable by immune system
How does immunotherapy in cancer tries to increase lymphocyte activity?
- Enhance factors that increase lymphocyte activation
- Inhibit factors that inhibit lmyphocyte activation

Which molecules are targeted in the immune-checkpoint blockade?
Explain how they work
- In activation of lymphocyte: inhibit inhibitory factors like
- CTLA4
- PDL-1/PD-1
- PDL1/B7.1
- In the recognition/killing phase (decrease inhibitory response)
- PDL-1/PD-1
- PDL1/B7.1
What are the Requirements for activation of an adaptive anti-tumour immune response?
What are the limitations involved in this?
- Local inflammation in the tumour (“danger signal”)
* tumor might first reach a certain size until signal sent - Expression and recognition of tumour antigens
* need to be present and different enough from own antigens
Explain the formation and role of inflammation in the immune respose against tumors
- Tumor growth on its own would not attract immune cells
- When tumor has reached a certain size –> inflammation
- recruits inate cells
- Recruits adaptive cells
Explain how immune responses to tumours have some similarities with those to virus infected cells
T-cells can sense abnormal proteins inside the cell via MHC I/II moleculse presentation (that present fragements of intracellular proteins)
What is a tumor-specific antigen?
Antigens that can only be found in tumor cells
What are the two types of Tumor-specific antigens?
Name examples for each
- Viral proteins
- tumor associated with certain viruses express the viral antigen on the surface
- E.g. EBC (Epstein Barr Virus)
- HPV (human papilloma virus)
- Mutated cellular proteins
- e.g. TGFß (Transforming Growth factor ß receptor III)
What is the difference between virus associated oppertunistic malignancies and viruses that cause malignancies in immunocompetnet individuals?
Name examples for each
In oppertunistic malignancies: in people who are immunosupressed infection might lead to malignancy
- EBV-positive lymphoma: Post-transplant immunosuppression
- HHV8-positive Kaposi sarcoma: HIV
Other viruses also cause cancer in normal, immunocompetent individuals
- HTLV1-associated leukaemia/lymphoma
- HepB virus- and HepC virus-associated hepatocellular carcinoma
- Human papilloma virus-positive genital tumours
Why does the HPV vaccine can also work therapeutically?
Because tumors express the viral antigens –> helps immune system to fight the tumor
Explain how an infection with HPV16 can cause cervical cancer
- Viral proteins E6+7 are oncoproteins
- They trigger aberrant cell growth and deregulation of cell cycle
*
Explain the mechanism the HPV vaccine works
- It has viral Surface proteins, incorporated into Virus-Like Particles (VLPs) that are presented to immune system
- Can protect the <1% of people that would naturally not be able to figh an infection with HPV and then would lead to neoplasms

What are tumor-associated antigens? (TAA)
Tumour-associated antigens (TAA) are normal cellular proteins which are aberrantly expressed (timing, location or quantity)
How can the immune system still fight TAAs though they are also present in normal tissue?
The immune system has to overcome tolerance to then protect them
Explain tumor associated antigens that are expressed at a wrong time
Name examples
- E.g. developmental antigens that should not be expressed in adult cells (except maybe placents/germ cells)
- . MAGE family: Melanoma associated antigens. Identified in melanoma also expressed in other tumours
- Carcinoembryonic antigen (CEA): normally only expressed in foetus/embryo, but overexpressed in a wide range of carcinomas
What kind of Protein is HER2?
How does it lead to cancer?
Human epidermal growth factor receptor 2
- is overexpressed in some breast cancers
- Increased stimmulation of proliferation
Overexpression of which protein is associated with Breast cancer?
HER2 Human epidermal growth factor receptor 2
Name some Tumor-associated antigens that are overexpressed in in cancers
- Human epidermal growth factor receptor 2 (HER2): overexpressed in some breast carcinomas
- Mucin 1 (MUC-1): membrane-associated glycoprotein, overexpressed in very many cancers
Explain the formation of central immunotolerance
It happens in the thymus during immune cell (T-cell) selection
- negative selection= destruction of auto-reactive cells
- positive selection= enhancing cells that can recognise MHC molecules
- this process is not perfect and some t-cells go into circulation and are auto-reactive –> tolerance
Which cells are tried to target in immunotherapy of TAAs?
T-lymphocytes that have escaped the selection and have some auto-reactivity
What are the limitations of Targeting of tumour-associated auto-antigens
for T cell-mediated immunotherapy of cancer
- 1.Auto-immune responses against normal tissues
- Immunological tolerance –> not having the t-cells to stimulate in the first place (because all auto-reactive cells have been destroyed)
* -Normal tolerance to auto-antigens
* -Tumour-induced tolerance
- Immunological tolerance –> not having the t-cells to stimulate in the first place (because all auto-reactive cells have been destroyed)
What are the different ways immunotherapy can be used to target cancer?
1) Antibody-based therapy
2) Therapeutic vaccination
3) Immune checkpoint blockade
4) Adoptive transfer of immune cells
5) Combinations of 1) to 4) above
Explain the use and different types of monochlonal antibody-based therapy in cancer treatment
- Naked
- just the antibody itself (e.g. anti HER2, anti EGFR, anti-CD20) –> neutralise
- Conjugated
- to enable specific delivery of
- radioacitve particle (e.g. anti CD20 linked to yttrium-90)
- drugs (anti HER2 linked to cytotoxic drug)
- to enable specific delivery of
- Bi-specific antibodies
- Genetically engineered to combine 2 specificities, e.g. anti CD3 and anti CD19 (Blinatumomab, approved for t cells targeting to destroy patients with B cell tumours)
Explain the use of therapeutic cancer vaccines
E.g. one used in treatment of advanced prostate cancer overall: enhanced immune response to cancer
- WBC are taken out from patient
- treated with fusion protein of TAA and a cytokine
- –> tought to enhance Dendtritic cell mediated T-cell activation
- Cells are injected back into patient
Explaint the engenering of a “personalised” tumor specific cancer vaccine
- DNA from normal cell and from cancer cell are extracted
- Two DNAs are compared to see if mutation can be found
- If mutation found: computer program predicts which mutated proteins will be presented on HLA on tumor
- Generation of new antibodies that target that specific antigens

Explain adoptive transfer of cells in immunotherapy of cancer
- Tumor or T-cells are extracted
- T-cells against cancer are cultrued
- may be genetically modified (e.g. by CAR)
- Transfered back to patient

What are CARs? How can they be used in cancer treatment?
Chimaeric Antigen Receptors (CARs)
- the variable region of an antibody is transfered to the normal T-cell protein
- if T-cell (with variable region on protein binds to the tumor antigen) -> it will activate the T-cell

Which Protein /Transcription factor is responsible for expression of auto-antigens in the Thymus?
AIRE (autoimmune regulator)


