Shapes Of Molecules Flashcards
What is this shape called?
How many bond pairs does it have?
How many lone pairs does it have?
What is its’ angle?

Linear.
2 bond pairs.
0 lone pairs.
180 degrees.
What is this shape called?
How many bond pairs does it have?
How many lone pairs does it have?
What is its’ angle?

Trigonal Planar.
3 bond pairs.
0 lone pairs.
120 degrees.
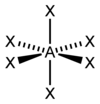
What is this shape called?
How many bond pairs does it have?
How many lone pairs does it have?
What is its’ angle?

Tetrahedral.
4 bond pairs.
0 lone pairs.
109.5 degrees.
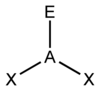
What is this shape called?
How many bond pairs does it have?
How many lone pairs does it have?
What is its’ angle?
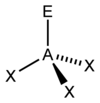
Pyramidal.
3 bond pairs.
1 lone pair.
107 degrees.
What is this shape called?
How many bond pairs does it have?
How many lone pairs does it have?
What is its’ angle?
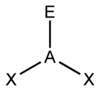
Non-Linear.
2 bond pairs.
2 lone pairs.
105.5 degrees.
What is this shape called?
How many bond pairs does it have?
How many lone pairs does it have?
What is its’ angle?
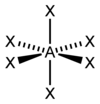
Octahedral.
6 bond pairs.
0 lone pairs.
90 degrees.
What shape is a linear molecule?

What shape is a Trigonal planar molecule?

What shape is a Tetrahedral molecule?

What shape is a Pyramidal molecule?

What shape is a Non-Linear molecule?
What shape is a Octahedral molecule?