MHD Flashcards
What is the relationship between Calveolin and Idiopathic Pulmonary Fibrosis?
Epithelial activation/injury leads to the release of TGF-B1 and this goes to block Caveolin in the fibroblasts. Caveolin normally functions to block deposition of collagen and extracellular matrix. W/o Caveolin, pulmonary fibrosis has a clearer path to development.
Anthracosis
Deposition of coal dust in the lungs
One form of Pneumoconioses (other examples include silicosis and asbestosis)
What is the key characteristic of sarcoidosis?
Non-caseating granulomas
What are Schaumann bodies and Asteroid Bodies, and what disease process are they associated with?
Schaumann: Concretions (hard masses) of calcium and protein
Asteroid: Star like stellate inclusions
Both found in SARCOIDOSIS
What changes in FEV1 w/ a bronchodilator defines reversibility?
12% and 200cc’s
99% of obstructions are _________ (upper/lower) airway.
Lower
Equation for Diffusion Capacity
DLCO = [CO]inhaled - [CO]exhaled
This is the difference between the CO inhaled and the CO exhaled, which tells you how much ended up diffusing into the blood.
If DLCO is low, but spirometry and lung volumes are normal, what lung issue might you suspect?
Pulmonary HTN
What are the (3) main components to a PFT and what do they each tell you?
- Spirometry (used to ID obstruction)
- Long Volume Determination (used to ID restriction)
- Diffusion Capcity Measurement (used to ID diffusion defect)
What are the (4) Categories for Asthma and what values define each (based on each criteria below):
*daytime symptoms/wk, nighttime symptoms/mo, FEV1 or PEFR, PEFR variability

RADS
Reactive Airway Dysfunction Syndrome
No prior asthma, then a “big-bang” exposure (ex. 9/11) leads to asthma which normally resolves in 6 months or so.
Samter’s Triad
A triad of symptoms which point to aspirin sensitivity:
- Asthma
- Nasal polyposis
- NSAID sensitivity
Allergic Bronchopulmonary Aspergillous
Asthma + Allergic response (+ skin test, IgE, eosinophils, etc.)
Stages of Sarcoidosis based on CXR
LAD = lymphadenopathy
ILD = Interstitial lung disease

What are your (3) main differentials when a pt presents w/ a cough?
- Postnasal drip
- Acid reflux
- Asthma
Fleischner Guidlines
What is it used for, and what are the guidlines?
Used for determining course of action after nodules found on low dose CT scan.

Identify the type of lung cancers being shown in each section
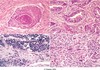
- Squamous cell
- Adenocarcinoma
- Small cell carcinoma
- Large cell undifferentiated carcinoma
Which bug is the most common cause for Community Acquired Pneumonia?
What are the clinical presentations associated with this bug (5)?
What does this bug look like?
Strep Pneumo
Acute onset fever, shaking chill, rusty sputum, shortness of breath, pleuritic chest pain
Lancet shaped diplococcus
ALL
Name the cytogenic markers which correlate with a good prognosis.
Name the cytogenic markers which correlate with a poor prognosis.
Good sign:
- t(12;21) [TEL1-AML1]
- Hyperdiploidy
Poor signs:
- t(9;22) [BCR-ABL] - Philidelphia chromosome
- t(4;11) [AF4-MLL]
Name the 4 WHO classifications of AML
What are the affliated cytogenic markers and what do they indicate?
- AML w/ recurrent genetic abnormalities
- t(15;17)PML/RARA good prognosis
- t(11q23;v)MLL poor prognosis
- AML arising from Myelodysplastic Syndrome (MDS) poor prognosis
- Therapy Related poor prognosis
- post chemo/radiation
- NOS intermediate prognosis
What is Myelodysplastic syndrome? What can it progress to?
It is also known as “preleukemia”
Hypercellular bone marrow which leads to a peripheral cytopenia and many morphological abnormalities. Often progresses to AML.
Poor prognosis
Acute Promyelocytic Anemia
What is it a type of? What is the genetic change? What does it increase risk of? How is it treated? What is the prognosis?
It is one of the classifications of AML
t(15;17)PML/RARA
There is fusion between the PML/Retinoic Acid Receptor genes creating an abnormal RAR.
Greatly increased risk of DIC due to the presence of MPO based auer rods.
Great prognosis if treated with all-trans retinoic acid (ATRA)
What is SIRS and what qualifications must be present for something to count as SIRS? (4 total options)
Systemic Inflammatory Response Syndrome
Massive inflammatory reaction from systemic cytokine release
At least 2 of the following must be present:
- Temperature >38° C or
- Heart rate >90
- Respiratory rate >20 or PaCO2
- WBC >12,000 or 10% immature
What are the (2) key cytokines in Sepsis Syndrome?
What 3rd cytokine has a key role?
TNFa and IL-1
IL-6 also plays a key role
List the (3) components of clinical shock
- Systemic arterial Hypotension
-
Clinical signs of hypoperfusion
- Skin (cold, clammy, etc.)
- Renal (oliguria)
- Neurologic (AMS)
- Hyperlactemia (due to decreased muscle perfusion)
What should be completed in first (3) hours of Sepsis Bundle? (4)
First (3) Hours:
- Measure lactate level
- Obtain blood cultures prior to administration of antibiotics
- Administer broad spectrum antibiotics
- Adminster 30 mL/kg crystalloid for hypotension or lactate > or equal to 4mmol/L
What should be completed within (6) hours of the Sepsis Bundle? (3)
- Apply vasopressor if it doesn’t respond to fluid resuscitation (to maintain MAP over 65 mmHg)
- Measure CVP and CV O2 sats if hypotension persists or initial lactate was high
- Remeasure lactate if initial lactate was elevated
When markers/factors correlate with a better prognosis for CLL/SLL? Worse?
Better: Mutations of the IGH gene
Worse: Richter syndrome/transformation to a higher process
Cytochemical staining for Hairy Cell Leukemia
Tartrate resistant acid phosphate (TRAP)
What is the cause for Adult T-cell Leukemia/Lymphoma? What kind of cells does it lead to and what is the prognosis?
HTLV-1
Usually fatal and leads to floret-like CD-4 cells
What is the key genetic change which leads to Follicular Lymphoma?
A t(14;18) BCL2/IGH fusion which leads to BCL2 over-amplification and an inability for apoptosis to occur as a result.
Key B cell markers?
T cell markers?
Myeloid markers?
B: CD19, CD20
T: CD3
Myeloid: Myeloperoxidase
Key genetic change in Mantle Cell Lymphoma
t (11;14) [Cyclin D1-IGH fusion] leading to cyclin D1 over-amplification and increased proliferation as a result.
Key translocations for Burkitt lymphoma
t(8;14) [MYC/IGH fusion]
Leads to MYC over-amplification and increased proliferation as a result
What are the 2 distinct subtypes of Diffuse Large B-cell lymphoma? What are the potential cytogenic features associated with diffuse large B-cell lymphoma?
Subtypes:
- Germinal Center B-cell (GCB): better prognosis
- Activated B-cell (ABC)
Cytogenic features:
- t(14;18)
- BCL6
- double hit: t(14; 18) + BCL6 (very poor prognosis)
What is the cytological change associated with CML? What does this lead to? What does the BM look like? How are the progentitors affected?
BCR-ABL fusion (philadelphia)
Early transformation leads to B and T cell involvement. This leads to a marked increase in neoplastic granulocytic precursors in the marrow.
Close to 100% BM cellularity
More megakaryocytes, fewer erythroid progenitors, and High basophil count
What is the mutation which leads to polycythemia vera?
JAK2 mutations
What are the mutations which lead to primary myelofibrosis (chronic idiopathic myelofibrosis)? What is the pathogenesis of this disease?
JAK2, CALR, and MPL point mutations
This disease is an early progression to obliterating marrow fibrosis. There are excessive collagen deposits in bone marrow due to secretion of PDGF and TGF-B by neoplastic megakaryocytes.
Normal marrow elements are displaced.
What are the mutations which cause essential thrombocytosis? What happens and what is the treatment.
Activating JAK2/MPL mutations. These lead to proliferation of the megakaryocytic lineage . Treated with aspirin.
Name the (3) Phases of ARDS
- Acute- early exudative phase
- Organizing- subacute proliferative phase
- Late- Fibrotic phase
Acute interstitial pneumonia
A catch-all term for a disease which presents like ARDS w/o any known etiology (thus ruling out ARDS). All declared after everything else has been ruled out.
Average time until patient dies = 2 months
What (2) diseases fall under the umbrella of COPD?
Emphysema and Chronic Bronchitis
Atopic vs Non-atopic Asthma
Atopic: Immune-mediated. Involves IgE and mast cells. Triggered by environmental allergens. + skin test.
Non-atopic: Non-immune triggering mechanisms (Hyperirritable bronchial tree; virus induced inflammation of the respiratory mucosa, etc.). - skin test
Name (2) of the key genes involved in asthma
- Chromosome 5q (has several susceptibility genes)
- ADAM 33-20q
(3) key histological findings associated with asthma
- Curshmann spirals (mucous plugs + whirls of shed epithelium)
- Eosinophils
- Charcot-leyden Crystals
Describe the pathophyiology of Chronic Bronchitis
What is the reid index and how does it factor in?
Hypertrophy of mucus secreting glands (goblet cell metaplasia). Leads to inflammation and fibrosis. Could result in cor pulmonale, infections, or carcinoma.
Reid index: gland:wall ratio. It is increased in CB.
Blue Bloater

